Correlation of Clinicopathologic Features of Filipino Primary Breast Cancer Patients with HER2 Subgroups Classified according to the ASCO/CAP 2018 Breast Cancer HER2 Testing Guidelines
DOI:
https://doi.org/10.21141/PJP.2023.18Keywords:
Breast cancer, ASCO/CAP, HER2, fluorescence in situ hybridization, immunohistochemistryAbstract
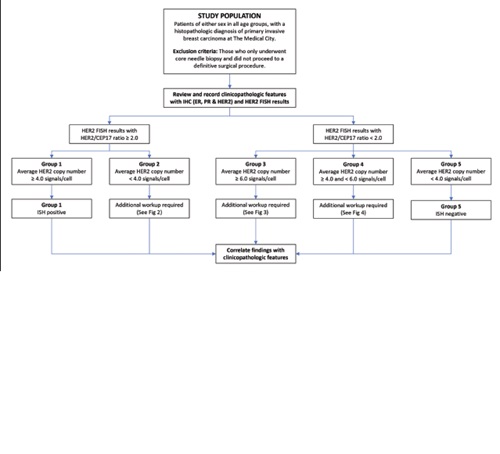
Background. Guidelines for testing human epidermal growth factor receptor 2 (HER2) in breast cancer using fluorescence in situ hybridization (FISH) were released in 2018. These guidelines were jointly developed by the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) to achieve a clearer designation of breast cancer HER2 status. Clinical correlation with other factors was also considered appropriate, especially for those cases classified under ISH groups 2, 3, and 4.
Objective. In this study, we examined clinicopathologic features among Filipino breast cancer patients whose HER2 status was reclassified based on the 2018 ASCO/CAP guidelines.
Methodology. One hundred and thirty-two (132) breast cancer cases with immunohistochemistry (IHC) equivocal results in the Medical City were enrolled from January 2017 up to December 2020. HER2 FISH results classified under groups 2, 3, and 4 were then re-evaluated for HER2-IHC status in accordance with the 2018 ASCO/CAP guidelines. The relationship between clinicopathologic features and HER2 status was analyzed using the Fisher exact test.
Results. Significant differences were found in histologic type, nuclear pleomorphism, mitotic rate, progesterone receptor (PR) status, and regional lymph node involvement among the reclassified ISH groups. In the conv+ group, the tumor cells did not involve the regional lymph nodes as compared to group 5, where the tumor cells were involved. The conv- group had a higher grade for nuclear pleomorphism, mitotic count, and overall Nottingham Histologic Grade than group 5. There was a significant association between progesterone receptors among the conv- group and group 1.
Conclusion. Filipino breast cancer cases whose HER2 status was reclassified to negative following the 2018 ASCO/CAP guidelines had statistically different clinicopathologic features from those classified as group 5.
Downloads
References
WHO Classification of Tumours Series, 5th ed, vol. 2. Breast tumours. Lyon, France: International Agency for Research on Cancer; 2019.
Eliyatkın N, Yalçın E, Zengel B, Aktaş S, Vardar E. Molecular classification of breast carcinoma: from traditional, old-fashioned way to a new age, and a new way. J Breast Health. 2015;11(2):59-66. https://pubmed.ncbi.nlm.nih.gov/28331693. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5351488. https://doi.org/10.5152/tjbh.2015.1669. DOI: https://doi.org/10.5152/tjbh.2015.1669
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-49. https://pubmed.ncbi.nlm.nih.gov/33538338. https://doi.org/10.3322/caac.21660. DOI: https://doi.org/10.3322/caac.21660
Azutillo E. Philippine-based Filipino women and breast cancer. Doctoral dissertation. Florida, USA: Nova Southeastern University; 2019. https://nsuworks.nova.edu/hpd_con_stuetd/67.
Argenal RN, Baterna ML, Peña CF, Ravina JN, Sionzon MR. Risk Factors of breast cancer among women: a meta analyses. 2019. [Slide show]. https://www.scribd.com/document/536638959/4-2-2-Risk-Factors-of-Breast-Cancer-among-Women-A-Meta-Analysis.
Gibson LJ, Héry C, Mitton N, et al. Risk factors for breast cancer among Filipino women in Manila. Int J Cancer. 2010;126(2):515-21. https://pubmed.ncbi.nlm.nih.gov/19626603. https://doi.org/ 10.1002/ijc.24769. DOI: https://doi.org/10.1002/ijc.24769
Elomina K, Cagampan MC. Predictive value of histologic characteristics on hormone receptor and HER-2 status of patients with invasive breast carcinoma, no special type, in an Academic Medical Center. Philip J Pathol. 2019;4(1):6-11. https://doi.org/10.21141/PJP.2019.02. DOI: https://doi.org/10.21141/PJP.2019.02
Orrantia-Borunda , Anchondo-Nuñez P, Acuña-Aguilar LE, Gómez-Valles FO, Ramírez-Valdespino CA. Subtypes of breast cancer. Breast Cancer. Brisbane, Australia: Exon Publications. 2022. DOI: https://doi.org/10.36255/exon-publications-breast-cancer-subtypes
Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11(2):263-75. https://pubmed.ncbi.nlm.nih.gov/21342044. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3092522. https://doi.org/10.1586/era.10.226 DOI: https://doi.org/10.1586/era.10.226
Kho MJN, Kho MR. Gene expression profiling for breast cancer in the philippines: real-world data on feasibility, possible disparities, and clinical implications for LMICs. J Clin Oncol. 2022;40(Suppl 16):e12569. https://doi.org/10.1200/JCO.2022.40.16_suppl.e12569 DOI: https://doi.org/10.1200/JCO.2022.40.16_suppl.e12569
Genuino AJ, Chaikledkaew U, Guerrero AM, Reungwetwattana T, Thakkinstian A. Cost-utility analysis of adjuvant trastuzumab therapy for HER2-positive early-stage breast cancer in the Philippines. BMC Health Serv Res. 2019;19(1):874. https://pubmed.ncbi.nlm.nih.gov/31752849. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6873585. https://doi.org/10.1186/s12913-019-4715-8. DOI: https://doi.org/10.1186/s12913-019-4715-8
Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/ College of American Pathologists Clinical Practice Guideline focused update. J Clin Oncol. 2018;36(20):2105–22. https://pubmed.ncbi.nlm.nih.gov/29846122. https://doi.org/10.1200/JCO.2018.77.8738. DOI: https://doi.org/10.1200/JCO.2018.77.8738
Wolff AC, Somerfield MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology–College of American Pathologists Guideline Update. Arch Pathol Lab Med. 2023;147(9):993–1000. https://pubmed.ncbi.nlm.nih.gov/37303228. https://doi.org/10.5858/arpa.2023-0950-SA. DOI: https://doi.org/10.5858/arpa.2023-0950-SA
Yang L, Chen M, Pu T, et al. The differences of clinicopathologic characteristics among subgroups of reclassified HER2 fluorescence in situ hybridization (FISH) according to the ASCO/CAP 2018 breast cancer HER2 testing guidelines. J Clin Pathol. 2020;73(5):283-90. https://pubmed.ncbi.nlm.nih.gov/31732618. https://doi.org/10.1136/jclinpath-2019-206222. DOI: https://doi.org/10.1136/jclinpath-2019-206222
Woo JW, Lee K, Chung YR, Jang MH, Ahn S, Park SY. The updated 2018 American Society of Clinical Oncology/College of American Pathologists guideline on human epidermal growth factor receptor 2 interpretation in breast cancer: comparison with previous guidelines and clinical significance of the proposed in situ hybridization groups. Hum Pathol. 2020;98:10–21. MID: 32027910. https://doi.org/10.1016/j.humpath.2020.01.003. DOI: https://doi.org/10.1016/j.humpath.2020.01.003
Munro AF, Twelves C, Thomas JS, Cameron DA, Bartlett JM. Chromosome instability and benefit from adjuvant anthracyclines in breast cancer. Br J Cancer. 2012;107(1):71-4. https://pubmed.ncbi.nlm.nih.gov/22644297. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3389422. https://doi.org/10.1038/bjc.2012.232. DOI: https://doi.org/10.1038/bjc.2012.232
Lee K, Kim HJ, Jang MH, Lee S, Ahn S, Park SY. Centromere 17 copy number gain reflects chromosomal instability in breast cancer. Sci Rep. 2019;9(1):17968. https://pubmed.ncbi.nlm.nih.gov/31784614. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6884473. https://doi.org/10.1038/s41598-019-54471-w. DOI: https://doi.org/10.1038/s41598-019-54471-w
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 PJP

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.








