Programmed Death Ligand 1 (PD-L1) Expression and its Association with Driver Mutations among Patients with Non-Small Cell Lung Cancer in a Private Tertiary Care Setting
DOI:
https://doi.org/10.21141/PJP.2022.17Keywords:
non-small cell lung cancer, programmed death ligand 1, PD-L1, driver mutationAbstract
Objective. The advent of immunotherapy has significantly changed the treatment and management of patients with advanced non-small cell lung cancer (NSCLC). Prior to initiation of immunotherapy,
evaluation of programmed death ligand 1 (PD-L1) expression is required. One factor that affects PD-L1 expression in NSCLC is the presence of oncogenic driver mutations; however, little data on its
association is available, especially in the Philippine setting. The study aims to determine the prevalence of PD-L1 expression and its association with driver mutations among patients with non-small cell lung cancer in a private tertiary care hospital in the Philippines.
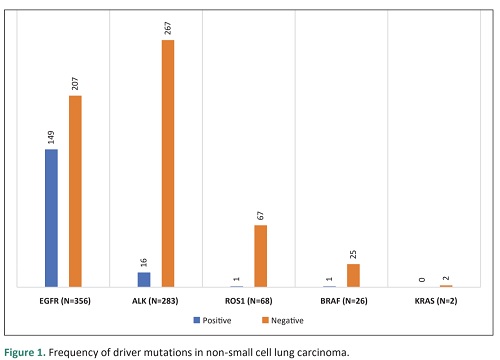
Methodology. The study was undertaken for a period of two years from July 2017-July 2019 at St. Luke’s Medical Center and included 446 NSCLC samples. PD-L1 was evaluated by immunohistochemistry using 22C3 anti-PD-L1 antibody clone, EnVision FLEX visualization system on Autostainer Link 48. Patient demographics and data on driver mutation testing were recorded. Statistical analysis was performed using logistic regression.
Results. PD-L1 expression was observed in 273 (61.20%) of 446 cases, 119 (61.20%) of which
demonstrated high PD-L1 expression while 154 (34.50%) had low PD-L1 expression. There was no
significant association between PD-L1 expression and EGFR mutation, ALK mutation, age, and gender.
For histologic type, high PD-L1 expression was significantly associated with adenocarcinoma and non-
small cell carcinoma, NOS.
Conclusion. The overall prevalence of PD-L1 expression in non-small cell lung carcinoma is 61.20%
based on the cases included. Although we did not find an association between PD-L1 expression and
EGFR and ALK mutation, our study observed that ALK-mutated NSCLCs have 4.7 odds of having high
PD-L1 expression, however, a higher sample size is warranted to truly determine significant association. The outcome of this study may provide help in the stratification of patients and predict those who will benefit from immunotherapy.
Downloads
References
2. Cancer Research UK. Lung cancer mortality statistics. 2016. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/mortality. Accessed April 28, 2019.
3. Department of Health. Philippine cancer control program. https://doh.gov.ph/philippine-cancer-control-program. Accessed April 25, 2019.
4. World Health Organization. Cancer Philippines 2020 country profile. 2020. https://www.who.int/publications/m/item/cancer-phl-2020.
5. Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288–300. https://pubmed.ncbi.nlm.nih.gov/27413711. PMCID: PMC4931124. https://doi.org/10.21037/tlcr.2016.06.07.
6. Garon EB, Rizvi NA, Leighl N, et al. Pembrolizumab for the treatment of non-small cell lung cancer. N Engl J Med. 2015;372(21):2018-28. https://pubmed.ncbi.nlm.nih.gov/25891174.https://doi.org/10.1056/NEJMoa1501824.
7. Teixidó C, Vilariño N, Reyes R, Reguart N. PD-L1 expression testing in non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:1758835918763493. https://pubmed.ncbi.nlm.nih.gov/29662547. PMCID:PMC5898658. https://doi.org/10.1177/1758835918763493.
8. Hunter KA, Socinski MA, Villaruz LC. PD-L1 testing in guiding patient selection for PD-1/PD-L1 inhibitor therapy in lung cancer. Mol Diagn Ther. 2018;22(1):1-10. https://pubmed.ncbi.nlm.nih.gov/29119407 PMCID:PMC5773410 DOI: 10.1007/s40291-017-0308-6.
9. Kim H, Chung JH. PD-L1 Testing in non-small cell lung cancer: past, present, and future. J Pathol Transl Med. 2019;53(4):199-206. https://pubmed.ncbi.nlm.nih.gov/31042863. PMCID: PMC6639705. https://doi.org/10.4132/jptm.2019.04.24.
10. Stratton MR, Campbell PJ, Futreal PA. The cancer genome. 2009;458(7239):719-24. https://pubmed.ncbi.nlm.nih.gov/19360079 PMCID: PMC2821689. https://doi.org/10.1038/nature07943.
11. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Version 3.2020. Non-small cell lung cancer. https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf. Accessed November 11, 2019.
12. Song Z, Yu X, Cheng G, Zhang Y. Programmed death-ligand 1 expression associated with molecular characteristic in surgically resected lung adenocarcinoma. J Transl Med. 2016;14(1):188. https://pubmed.ncbi.nlm.nih.gov/27342566. PMCID: PMC4919857. https://doi.org/10.1186/s12967-016-0943-4.
13. El-Telbany A, Ma PC. Cancer genes in lung cancer racial disparities: are there any? Genes Cancer. 2012; 3(7-8):467–80. https://pubmed.ncbi.nlm.nih.gov/23264847. PMCID: PMC3527990. https://doi.org/10.1177/1947601912465177.
14. Hirsch FR, Varella-Garcia M, Bunn PA Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21(20):3798-807. https://pubmed.ncbi.nlm.nih.gov/12953099. https://doi.org/10.1200/JCO.2003.11.069.
15. Nee-Estuye-Evangelista CK, Andal JL, Ang DC. Frequency of epidermal growth factor receptor mutations among filipino patients with non-small cell lung carcinoma. Philipp J Pathol. 2018;3(1):6-11. https://doi.org/10.21141/PJP.2018.002.
16. Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol. 2018;36(16):1631-41. https://pubmed.ncbi.nlm.nih.gov/29504847. https://doi.org/10.1200/JCO.2017.76.8671.
17. Shaw AT, Solomon B. UpToDate. 2019. Anaplastic lymphoma kinase (ALK) fusion oncogene positive in non-small cell lung cancer. https://www.uptodate.com/contents/anaplastic-lymphoma-kinase-alk-fusion-oncogene-positive-non-small-cell-lung-cancer#H1. Accessed November 11, 2019.
18. Vijayalkshmi R, Krishnamurthy A. Targetable “driver” mutations in non-small cell lung cancer. Indian J Surg Oncol. 2011; 2(3):178–88. https://pubmed.ncbi.nlm.nih.gov/22942608. PMCID: PMC3272176. https://doi.org/10.1007/s13193-011-0108-0.
19. Rimkunas VM, Crosby KE, Li D, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res.2012;18(16):4449-57. https://pubmed.ncbi.nlm.nih.gov/22661537. https://doi.org/10.1158/1078-0432.CCR-11-3351.
20. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863-70. https://pubmed.ncbi.nlm.nih.gov/22215748. PMCID: PMC3295572. https://doi.org/10.1200/JCO.2011.35.6345.
21. Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29(15):2046-51. https://pubmed.ncbi.nlm.nih.gov/21483012. PMCID:PMC3107760. https://doi.org/10.1200/JCO.2010.33.1280.
22. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585-93. https://pubmed.ncbi.nlm.nih.gov/27225694.PMCID: PMC5026567. https://doi.org/10.1158/1078-0432.CCR-15-3101.
23. Yang H, Chen H, Luo S, et al. The correlation between programmed death ligand 1 expression and driver gene mutations in NSCLC. Oncotarget. 2017; 8(14): 23517–28. https://pubmed.ncbi.nlm.nih.gov/28423587.
PMCID: PMC5410323. https://doi.org/10.18632/oncotarget.15627.
24. Lan B, Ma C, Zhang C, Chai S, et al. Association between PD-L1 expression and driver gene status in non-small cell lung cancer: a meta-analysis. Oncotarget. 2018 ;9(7):7684-99. https://pubmed.ncbi.nlm.nih.gov/29484144. PMCID: PMC5800936. https://doi.org/10.18632/oncotarget.23969.
25. Adiaon B. Immunotherapy: A bright future for cancer treatment. The Philippine Star. 12 Sep 2017. https://www.pressreader.com/philippines/the-philippine-star/20170912/282638917745551. Accessed November 11, 2019.
26. Gadgeel SM, Garassino MC, Esteban E, et al. KEYNOTE-189: Updated OS and progression after the next line of therapy (PFS2) with pembrolizumab (pembro) plus chemo with pemetrexed and platinum vs placebo plus chemo for metastatic nonsquamous NSCLC. J Clin Oncol.2019;37(15). https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.9013.
27. Azuma, K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR-mutations in surgically resected non-small cell lung cancer. Ann Oncol. 2014;25(10):1935-40. https://pubmed.ncbi.nlm.nih.gov/25009014. https://doi.org/10.1093/annonc/mdu242.
28. D’Inecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small cell lung cancer patients. Br J Cancer. 2015;112(1):95-102. https://pubmed.ncbi.nlm.nih.gov/25349974. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4453606. https://doi.org/10.1038/bjc.2014.555.
29. Inoue Y, Yoshimura K, Mori K, et al. Clinical significance of PDL1 and PDL2 copy number grains in non-small-cell lung cancer. Oncotarget. 2016;7(22):32113-28. https://pubmed.ncbi.nlm.nih.gov/27050074. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5078001. https://doi.org/10.18632/oncotarget.8528.
30. Cha YJ, Kim HR, Lee CY, Cho BC, Shim HS. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer. 2016;97:73-80. https://pubmed.ncbi.nlm.nih.gov/27237031. https://doi.org/10.1016/j.lungcan.2016.05.001.
31. Takada K, Okamoto T, Shoji F, et al. Clinical significance of PD-L1 protein expression in surgically resected lung adenocarcinomas. J Thorac Oncol. 2016;11(11):1879-90. https://pubmed.ncbi.nlm.nih.gov/27346415. https://doi.org/10.1016/j.jtho.2016.06.006.
32. Chen Q, Fu YY, Yue QN, et al. Distribution of PD-L1 expression and its relationship with clinicopathologic variables: an audit from 1071 cases of surgically resected non-small cell lung cancer. Int J Clin Exp Pathol. 2019;12(3):774-86. https://pubmed.ncbi.nlm.nih.gov/31933885. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6945142.
33. Grosso J, Horak CE, Inzunza D, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol. 2013;31(Suppl 15): Abstract 3016.
34. Davar D, Socinski MA, Dacic S, Burns TF. Near complete response after single dose of nivolumab in patient with advanced heavily pre-treated KRAS-mutant pulmonary adenocarcinoma. Exp Hematol Oncol. 2015;4:34. https://pubmed.ncbi.nlm.nih.gov/26673119. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4678465. https://doi.org/10.1186/s40164-015-0029-7.
35. Hong S, Chen N, Fang W, et al. Upregulation of PD-L1 by EML4–ALK fusion protein mediates the immune escape in ALK-positive NSCLC: implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology. 2015;5(3):e1094598. https://pubmed.ncbi.nlm.nih.gov/27141355. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4839382. https://doi.org/10.1080/2162402X.2015.1094598.
36. Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 expression by the EML4–ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21(17):4014-21. https://pubmed.ncbi.nlm.nih.gov/26019170. https://doi.org/10.1158/1078-0432.CCR-15-0016.
37. Bassanelli M, Sioletic S, Martini M, et al. Heterogeneity of PD-L1 expression and relationship with biology of NSCLC. Anticancer Res. https://pubmed.ncbi.nlm.nih.gov/29970498. https://doi.org/10.21873/anticanres.12662.
38. Aggarwal C, Rodriguez AD, Felip E, et al. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024. Ann Oncol.2016;27(Suppl 6):VI363. https://doi.org/10.1093/annonc/mdw378.14.
39. Holmes M, Mahar A, Lum T, Boyer M, Kao S, Cooper W. Prevalence of PD-L1 expression rates in different NSCLC Specimens. J Thoracic Oncol. 2019;14(10).
40. Chang YC, Hsu PC, Li SH, et al. The prevalence of PD-L1 Expression in Lung Cancer. Clin Oncol. 2019;4(1):1591. https://www.clinicsinoncology.com/open-access/the-prevalence-of-pd-l1-expression-in-lung-cancer-1745.pdf.
41. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9): 2892–11. https://pubmed.ncbi.nlm.nih.gov/26609494. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4633915.
42. Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011; 30(5):287–92. https://pubmed.ncbi.nlm.nih.gov/21527061. https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC4013393. https://doi.org/10.5732/cjc.011.10106.
43. Cooper, WA, Tran T, Villain RE, et al. PD-L1 expression is favorable prognostic factor in early-stage non-small cell lung carcinoma. Lung Cancer. 2015;89(2):181-8. https://pubmed.ncbi.nlm.nih.gov/26024796. https://doi.org/10.1016/j.lungcan.2015.05.007.
44. Schmidt LH, Kummel A, Görlich D, et al. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS One. 2015;10(8):e0136023. https://pubmed.ncbi.nlm.nih.gov/26313362. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4552388. https://doi.org/10.1371/journal.pone.0136023.
45. Tang Y, Fang W, Zhang Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6(16):14209-19. https://pubmed.ncbi.nlm.nih.gov/25895031. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4546461. https//doi.org/10.18632/oncotarget.3694.
46. Zhang, M, Li G, Wang Y. et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017;7(1):10255. https://pubmed.ncbi.nlm.nih.gov/28860576. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5578960. https://doi.org/10.1038/s41598-017-10925-7.
47. Li H, Yangyang X, Wan B, et al. The clinicopathological and prognostic significance of PD-L1 expression assessed by immunohistochemistry in lung cancer: a meta-analysis of 50 studies with 11,383 patients. Transl Lung Cancer Res. 2019;8(4):429–49. https://pubmed.ncbi.nlm.nih.gov/31555517. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6749117. https://doi.org/10.21037/tlcr.2019.08.04.
48. Bai Y, Chen X, Hou L, et al. PD-L1 expression and its effect on clinical outcomes of EGFR-mutant NSCLC patients treated with EGFR-TKIs. Cancer Biol Med. 2018;15(4):434–42. https://pubmed.ncbi.nlm.nih.gov/30766753. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6372913. https://doi.org/10.20892/j.issn.2095-3941.2018.0223.
49. Miyazawa T, Marushima H, Saji H. et al. PD-L1 expression in non-small-cell lung cancer including various adenocarcinoma subtypes. Ann Thorac Cardiovasc Surg. 2019;25(1):1-9. https://pubmed.ncbi.nlm.nih.gov/30282880. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6388302. https://doi.org/10.5761/atcs.oa.18-00163.
50. Okita R, Maeda A, Shimizu K, Nojima Y, Saisho S, Nakata M. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother. 2017;66(7):865-76. https://pubmed.ncbi.nlm.nih.gov/28341875. https://doi.org/10.1007/s00262-017-1986-y.
51. Midha A, Sharpe A, Scott M, et al. D-L1 expression in advanced NSCLC: primary lesions versus metastatic sites and impact of sample age. J Clin Oncol. 2016; 34(15):Suppl 3025. https://doi.org/10.1200/JCO.2016.34.15_suppl.3025.
52. Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 2014;12(1):3-13. https://pubmed.ncbi.nlm.nih.gov/24442106. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3925633. https:/doi.org/10.1158/1541-7786.MCR-13-0539.
53. Skov BG, Rørvig SB, Jensen THL, Skov T. The prevalence of programmed death ligand-1 (PD-L1) expression in non-small cell lung cancer in an unselected, consecutive population. Mod Pathol. 2020;33(1):109-17. https://pubmed.ncbi.nlm.nih.gov/31383957. https://doi.org/10.1038/s41379-019-0339-0.
54. Mu CY, Huang JA, Chen Y, Chena C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor-infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682-688. https://pubmed.ncbi.nlm.nih.gov/20373055. https://doi.org/10.1007/s12032-010-9515-2.
55. Janzic U, Kern I, Janzic A, Cavka L, Cufer T. PD-L1 Expression in squamous-cell carcinoma and adenocarcinoma of the lung. Radiol Oncol. 2017;51(3):357-62. https://pubmed.ncbi.nlm.nih.gov/28959173. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5612001. https://doi.org/10.1515/raon-2017-0037.
56. Scheel AH, Ansén S, Schultheis AM, et al. PD-L1 expression in non-small cell lung cancer: Correlations with genetic alterations. Oncoimmunology. 2016;5(5):e1131379. https://pubmed.ncbi.nlm.nih.gov/27467949. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4910698. https://doi.org/10.1080/2162402X.2015.1131379.
57. Brody R, Zhang Y, Ballas M, et al. PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer. 2017;112:200-15. https://pubmed.ncbi.nlm.nih.gov/29191596. https://doi.org/10.1016/j.lungcan.2017.08.005.
58. Righi L, Vavalà T, Rapa I. et al. Impact of non-small cell lung cancer-not otherwise specified immunophenotyping on treatment outcome. J Thorac Oncol. 2014;9(10):1540-6. https://pubmed.ncbi.nlm.nih.gov/25521399. https://doi.org/10.1097/JTO.0000000000000271
59. Munari E, Zamboni G, Marconi M, et al. PD-L1 expression heterogeneity in non-small cell lung cancer: evaluation of small biopsies reliability. Oncotarget. https://pubmed.ncbi.nlm.nih.gov/29163815. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5685736. https://doi.org/10.18632/oncotarget.21485.
60. Hwang DM, Albaqer T, Santiago RC, et al. Prevalence and heterogeneity of PD-L1 expression by 22C3 assay in routine population-based and reflexive clinical testing in lung cancer. J Thorac Oncol. 2021;16(9):1490-1500. https://pubmed.ncbi.nlm.nih.gov/33915250. https://doi.org/10.1016/j.jtho.2021.03.028.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 PJP

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.








