Prevalence of CKIT and PDGFRA Mutation in Gastrointestinal Stromal Tumors among Filipinos
DOI:
https://doi.org/10.21141/10.21141/PJP.2022.16Keywords:
Gastrointestinal Stromal Tumors, GIST, sequencing, CD117, CKIT, PDGFRAAbstract
Background: Gastrointestinal stromal tumors (GIST) is defined as specific, typically kit (CD117)-positive and CKIT or platelet-derived growth factor receptor alpha (PDGFRA) mutation-driven mesenchymal tumors that can occur anywhere in the GI tract. GIST diagnosis relies heavily on immunohistomorphology. However, with the advent of molecular testing, the classification, diagnosis and targeted-therapy for gastrointestinal mesenchymal tumors have been greatly improved. In the Philippines, molecular testing is not yet readily available as in other countries. The local molecular profile of gastrointestinal stromal tumors is a point of investigation as treatment may be more tailored to the patients’ needs.
Objective: This study aims to determine the prevalence of CKIT and PDGFRA mutations among formalin-fixed and paraffin embedded gastrointestinal stromal tumors and other gastrointestinal mesenchymal tumors in St. Luke’s Medical Center – Quezon City.
Methods: A retrospective cross-sectional study of formalin fixed and paraffin embedded tumor samples diagnosed as Gastrointestinal Stromal Tumor from January 1, 2009 to December 31, 2017 will be analyzed for KIT and PDGFRA mutations.
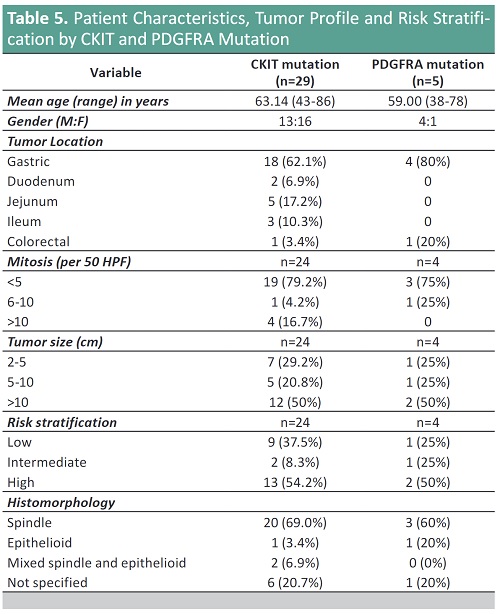
Result: The epidemiology of GIST remains constant in that mean age group is the 5th to 6th decade, with equal gender distribution, and stomach followed by small bowel are the most common sites.
Mutational analysis of the GISTs showed predominantly KIT Exon 11 (47.83%) followed by CKIT Exon 9 (13.04%) and PDGFRA Exon 18 (10.87%). For KIT Exon 11, deletion is the most common mutations followed by point mutations. No mutation is detected in 47.83% of GISTs.
Conclusion: Mutational analysis for CKIT-PDGFRA is warranted among GIST patients, as it may significantly influence treatment protocol in our patients.
Downloads
References
2. Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumor: the incidence, prevalence, clinical course and prognostication in the preimatinib mesylate ear – a population-based study in western Sweden. Cancer. 2005;103(4):821-9. https://pubmed.ncbi.nlm.nih.gov/15648083. https://doi.org/10.1002/cncr.20862.
3. Xu Z, Huo X, Tang C, Ye Hua, et al. Frequent KIT mutations in human gastrointestinal stromal tumors. Sci Rep. 2014;4:5907. https://pubmed.ncbi.nlm.nih.gov/25080996. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4118194. https://doi.org/10.1038/srep05907.
4. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51-8. https://pubmed.ncbi.nlm.nih.gov/10636102.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1420965. https://doi.org/10.1097/00000658-200001000-00008.
5. Chou FF, Eng HL, Sheen-Chen SM. Smooth muscle tumors of the gastrointestinal tract: analysis of prognostic factors. Surgery.1996;119(2):171-7. https://pubmed.ncbi.nlm.nih.gov/8571202. https://doi.org/10.1016/s0039-6060(96)80165-6.
6. Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100(1):162-8. https://pubmed.ncbi.nlm.nih.gov/15654796. https://doi.org/10.1111/j.1572-0241.2005.40709.x.
7. Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30(1):90-6. https://pubmed.ncbi.nlm.nih.gov/16330947. https://doi.org/10.1097/01.pas.0000176433.81079.bd.
8. Mussi C, Schildhaus HU, Gronchi A, Wardelmann E, Hohenberger P. Therapeutic consequences from molecular biology for gastrointestinal stromal tumor patients affected by neurofibromatosis type 1. Clin Cancer Res. 2008;14(14):4550-5. https://pubmed.ncbi.nlm.nih.gov/18628470. https://doi.org/10.1158/1078-0432.CCR-08-0086.
9. Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23(4):377-89. https://pubmed.ncbi.nlm.nih.gov/10199467. https://doi.org/10.1097/00000478-199904000-00002.
10. Joensuu H. Gastrointestinal stromal tumor (GIST). Ann Oncol. 2006;17(Suppl 10):x280-6. https://pubmed.ncbi.nlm.nih.gov/17018739. https://doi.org/10.1093/annonc/mdl274.
11. Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: the Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005;117(2):289–93. https://pubmed.ncbi.nlm.nih.gov/15900576. https://doi.org/10.1002/ijc.21167.
12. Minzhi L, Wu C, Zheng Y, Zhao N. Incidence and survival analysis of gastrointestinal stromal tumors in shanghai: a population-based study from 2001 to 2010. Gastroenterology Res Pract. 2014;2014:834136. https://pubmed.ncbi.nlm.nih.gov/24864136. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4017880. https://doi.org/10.1155/2014/834136.
13. Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152(5):1259-69. https://pubmed.ncbi.nlm.nih.gov/9588894. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1858579.
14. Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11(8):728-34. https://pubmed.ncbi.nlm.nih.gov/9720500.
15. Zhao X, Yue C. Gastrointestinal stromal tumor. J Gastrointest Oncol. 2012;3(3):189–208. https://pubmed.ncbi.nlm.nih.gov/22943011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3418531. https://doi.org/10.3978/j.issn.2078-6891.2012.031.
16. Gajiwala K, Wu, J, Christensen J, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009;106(5):1542–7. https://pubmed.ncbi.nlm.nih.gov/19164557. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2635778. https://doi.org/10.1073/pnas.0812413106.
17. Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315(3):971-9. https://pubmed.ncbi.nlm.nih.gov/16002463. https://doi.org/10.1124/jpet.105.084145.
18. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-78. https://pubmed.ncbi.nlm.nih.gov/22089421. https://doi.org/10.1038/nrc3143.
19. Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7(1):22. https://pubmed.ncbi.nlm.nih.gov/33737510. https://doi.org/10.1038/s41572-021-00254-5.
20. Lux ML, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol 2000;156:791-5. https://pubmed.ncbi.nlm.nih.gov/10702394. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1876850. https://doi.org/10.1016/S0002-9440(10)64946-2.
21. Oppelt PJ, Hirbe AC, Van Tine BA. Gastrointestinal stromal tumors (GISTs): point mutations matter in management, a review. J Gastrointest Oncol. 2017;8(3):466–73. https://pubmed.ncbi.nlm.nih.gov/28736634. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5506287. https://doi.org/10.21037/jgo.2016.09.15.
22. NCCN. Clinical Practice Guidelines in Oncology v.2.2022. Soft tissue sarcoma. Accessed 2012. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1464.
23. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of Experts. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v98-102. https://pubmed.ncbi.nlm.nih.gov/20555113. https://doi.org/10.1093/annonc/mdq208.
24. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39(10):1411-9. https://pubmed.ncbi.nlm.nih.gov/18774375. https://doi.org/10.1016/j.humpath.2008.06.025.
25. Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. https://pubmed.ncbi.nlm.nih.gov/26618334. https://doi.org/10.1016/j.canep.2015.10.031.
26. Yamamoto H, Oda Y. Gastrointestinal stromal tumor: recent advances in pathology and genetics. Pathol Int. 2014;65(1):9-18. https://pubmed.ncbi.nlm.nih.gov/25414046. https://doi.org/10.1111/pin.12230.
27. Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical and molecular genetic study of seventeen cases and comparison with esophageal leiomyomas and leiomyosarcoma. Am J Surg Pathol. 2000;24(2):211–22. https://pubmed.ncbi.nlm.nih.gov/10680889. https://doi.org/10.1097/00000478-200002000-00007.
28. Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008; 3:557-86. https://pubmed.ncbi.nlm.nih.gov/18039140. https://doi.org/10.1146/annurev.pathmechdis.3.121806.151538.
29. Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016; 19(1):3-14. https://pubmed.ncbi.nlm.nih.gov/26276366. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4688306. https://doi.org/10.1007/s10120-015-0526-8.
30. Patil DP, Rubin P. Gastrointestinal stromal tumor: advances in diagnosis and management. Arch Pathol Lab Med. 2011;135(10):1298-310. https://pubmed.ncbi.nlm.nih.gov/21970485. https://doi.org/10.5858/arpa.2011-0022-RA.
31. Espinosa I, Lee CH, Kim MK, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32(2):210-8. https://pubmed.ncbi.nlm.nih.gov/18223323. https://doi.org/10.1097/PAS.0b013e3181238cec.
32. Miettinen M, Zeng-Feng W, Lasota J. DOG1 Antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009;33(9):1401-8. https://pubmed.ncbi.nlm.nih.gov/19606013. https://doi.org/10.1097/PAS.0b013e3181a90e1a.
33. West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165(1):107-13. https://pubmed.ncbi.nlm.nih.gov/15215166. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1618538. https://doi.org/10.1016/S0002-9440(10)63279-8.
34. Nishida T. Asian consensus guidelines for gastrointestinal stromal tumor: what is the same and what is different from global guidelines. Transl Gastroenterol Hepatol. 2018;3:11. https://pubmed.ncbi.nlm.nih.gov/29552662. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5847913. https://doi.org/10.21037/tgh.2018.01.07.
35. Szucs Z, Thway K, Fisher C, et al. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017;13(1):93-107. https://pubmed.ncbi.nlm.nih.gov/27600498. https://doi.org/10.2217/fon-2016-0192.
36. Wozniak A, Rutkowski P, Piskorz A et al. Polish Clinical GIST Registry. Prognostic value of KIT/PDGFRA mutations in gastrointestinal stromal tumours (GIST): Polish Clinical GIST Registry experience. Ann. Oncol. 2012;23(2):353–60. https://pubmed.ncbi.nlm.nih.gov/21527588. https://doi.org/10.1093/annonc/mdr127.
37. Huss S, Künstlinger H, Wardelmann E, et al. A subset of gastrointestinal stromal tumours previously regarded as wild-type tumours carries somatic activating mutations in KIT exon 8 (p.D419del). Mod. Pathol. 2013;26(7):1004–12. https://pubmed.ncbi.nlm.nih.gov/23599150. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3701292. https://doi.org/10.1038/modpathol.2013.47.
38. Künstlinger H, Huss S, Merkelbach-Bruse S, et al. Gastrointestinal stromal tumours with KIT exon 9 mutations: update on genotype–phenotype correlation and validation of a high-resolution melting assay for mutational testing. Am J Surg Pathol. 2013;37(11):1648–59. https://pubmed.ncbi.nlm.nih.gov/24061512. https://doi.org/10.1097/PAS.0b013e3182986b88.
39. Mulet-Margalef N, Garcia-Del-Muro X. Sunitinib in the treatment of gastrointestinal stromal tumor: patient selection and perspectives. Onco Targets Ther. 2016;9:7573-82. https://pubmed.ncbi.nlm.nih.gov/28008275. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5171199. https://doi.org/10.2147/OTT.S101385.
40. Arceno J, Chua K, Lo R, et al. Platelet-derived growth factor receptor-alpha D842v mutation in a spindle cell type gastrointestinal stromal tumor: a case report. Philipp J Pathol. 2018;3(1):16-9. https://doi.org/10.21141/PJP.2018.004.
41. Li K, Cheng H, Li Z, et al. Genetic progression in gastrointestinal stromal tumors: mechanisms and molecular interventions. Oncotarget. 2017;8(36):60589-604. https://pubmed.ncbi.nlm.nih.gov/28947997. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5601165. https://doi.org/10.18632/oncotarget.16014.
42. Lai S, Wang G, Cao X, et al. KIT over-expression by p55PIK-PI3K leads to imatinib-resistance in patients with gastrointestinal stromal tumors. Oncotarget. 2016; 7(2):1367-79. https://pubmed.ncbi.nlm.nih.gov/26587973. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4811466. https://doi.org/10.18632/oncotarget.6011.
43. Antonescu CR, DeMatteo RP. CCR 20th anniversary commentary: a genetic mechanism of imatinib resistance in gastrointestinal stromal tumor-where are we a decade later? Clin Cancer Res. 2015; 21(15):3363-5. https://pubmed.ncbi.nlm.nih.gov/26240289. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4526110. https://doi.org/10.1158/1078-0432.CCR-14-3120.