Breast Panel Biomarker Changes After Neoadjuvant Chemotherapy in Breast Cancer
A Single-Center Study
DOI:
https://doi.org/10.21141/PJP2024.08Keywords:
residual invasive breast carcinoma, neoadjuvant systemic therapy, breast panelAbstract
Objectives. The aim of this study is to evaluate the breast panel biomarker changes and tumor intrinsic subtype after neoadjuvant chemotherapy among patients with residual invasive breast carcinoma whose breast specimens were processed at St. Luke’s Medical Center - Quezon City SLMC-QC) from 1 January 2017 to 30 June 2023.
Methodology. Cases of residual invasive breast carcinoma status post neoadjuvant systemic therapy were identified by retrospective review of cases. The baseline characteristics, type of biopsy and resection procedures, pre – and post–neoadjuvant ER, PR and HER2 status and pre – and post–neoadjuvant tumor intrinsic subtype were analyzed using frequency and percentage. The comparison of the changes in pre- and post-neoadjuvant breast panel biomarkers were analyzed by using McNemar test while the changes in the intrinsic tumor subtype was done using Wilcoxon signed-rank test.
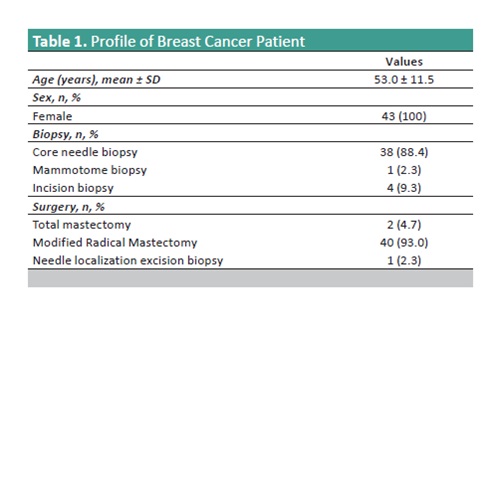
Results. This study encompassed a total of 43 cases of residual invasive breast carcinoma following neoadjuvant systemic therapy. The data disclosed shifts in the breast molecular profile and intrinsic subtype post-administration of neoadjuvant systemic therapy. The alterations in hormone receptor status, ER and PR, were observed in 11.6% of cases, while HER-2 status exhibited changes in 2.3%. A 14% change in the tumor intrinsic subtype is observed. Among the initial 18 Luminal A cases, 1 transitioned to Luminal B, and among the 6 Luminal B cases, 2 become HER2 enriched subtypes. Furthermore, among the initial 12 HER2 enriched cases, three shifted to Luminal B, while all triple-negative cases remained unchanged after chemotherapy.
Conclusion. Based on our findings, alterations in the molecular profile of breast tumors, including shifts in intrinsic subtype after neoadjuvant chemotherapy (NAC), could impact patient prognosis. While the data generated from this study may not exhibit statistical significance, its clinical relevance is noteworthy. In summary, retesting of breast biomarkers in the resection specimen is recommended to accurately ascertain the appropriate use of targeted therapy.
Downloads
References
Jabbout M, Massad C, Boulos F. Variability in hormone and growth factor receptor expression in primary versus recurrent, metastatic and post-neoadjuvant breast carcinoma. Breast Cancer Res Treat. 2012;135(1):29–37. https://pubmed.ncbi.nlm.nih.gov/22484731. https://doi.org/10.1007/s10549-012-2047-z. DOI: https://doi.org/10.1007/s10549-012-2047-z
Zhang N, Moran M, Huo Q, Haffty B, Yang Q. The hormonal receptor status in breast cancer can be altered by neoadjuvant chemotherapy: a meta-analysis. Cancer Invest.2011;29(9):594–8. https://pubmed.ncbi.nlm.nih.gov/22011281. https://doi.org/10.3109/07357907.2011.621913. DOI: https://doi.org/10.3109/07357907.2011.621913
Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol. 2015; 28(9): 1185–201. https://pubmed.ncbi.nlm.nih.gov/26205180. https://doi.org/10.1038/modpathol.2015.74. DOI: https://doi.org/10.1038/modpathol.2015.74
Lanjewar S, Patil P, Fineberg S. Pathologic reporting practices for breast cancer specimens after neoadjuvant chemotherapy—a survey of pathologists in academic institutions across the United States. Mod Pathol. 2020; 33(1):91–8. https://pubmed.ncbi.nlm.nih.gov/31383962. https://doi.org/10.1038/s41379-019-0326-5 DOI: https://doi.org/10.1038/s41379-019-0326-5
Sahoo S, Krings G, Chen YY, et al. Standardizing pathologic evaluation of breast carcinoma after neoadjuvant chemotherapy. Arch Pathol Lab Med. 2022;147(5):591-603. https://pubmed.ncbi.nlm.nih.gov/35976643. https://doi.org/10.5858/arpa.2022-0021-EP. DOI: https://doi.org/10.5858/arpa.2022-0021-EP
National Comprehensive Cancer Network. NCCN Guidelines Invasive Breast Cancer. https://www.nccn.org/patients/guidelines/content/PDF/breast-invasive-patient.pdf. Accessed December 9, 2023.
Lim SK, Lee MH, Park IH, et al. Impact of molecular subtype conversion of breast cancers after neoadjuvant chemotherapy on clinical outcome. Cancer Res Treat. 2016;48(1):133–41. https://pubmed.ncbi.nlm.nih.gov/25865655. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4720061. https://doi.org/10.4143/crt.2014.262. DOI: https://doi.org/10.4143/crt.2014.262
Xian Z, Quinones AK, Tozbikian G, Zynger DL. Breast cancer biomarkers before and after neoadjuvant chemotherapy: does repeat testing impact therapeutic management? Hum Pathol. 2017;62:215–21. https://pubmed.ncbi.nlm.nih.gov/28041972. https://doi.org/10.1016/j.humpath.2016.12.019.
Gahlaut R, Bennett A, Fatayer H, et al. Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression—implications for the practicing oncologist. Eur J Cancer. 2016;60:40–8. https://pubmed.ncbi.nlm.nih.gov/27062316. https://doi.org/10.1016/j.ejca.2016.03.006. DOI: https://doi.org/10.1016/j.ejca.2016.03.006
Xian Z, Quinones AK, Tozbikian G, Zynger DL. Breast cancer biomarkers before and after neoadjuvant chemotherapy: does repeat testing impact therapeutic management? Hum Pathol. 2017; 62:215–21. https://pubmed.ncbi.nlm.nih.gov/28041972. https://doi.org/10.1016/j.humpath.2016.12.019. DOI: https://doi.org/10.1016/j.humpath.2016.12.019
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 PJP

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.








