SARS-CoV-2 RT-PCR Ct Value and Laboratory Tests
Clinicopathologic Characteristics among Adult Filipino Inpatients diagnosed with COVID-19 in a Tertiary Medical Center
DOI:
https://doi.org/10.21141/PJP.2023.07Abstract
Introduction. The role of the laboratory during the COVID-19 pandemic is not limited to just diagnosis of the disease, but also in clinical decision-making, by providing information on relevant laboratory biomarkers. Clinicians also use Ct value to guide patient management. There are limited studies available locally regarding the significance of Ct value and pertinent laboratory biomarkers in COVID-19 patients. This study aimed to assess the aforementioned laboratory data, along with the clinicopathologic characteristics of affected patients, and determined if this information may be useful for robust clinical decision-making.
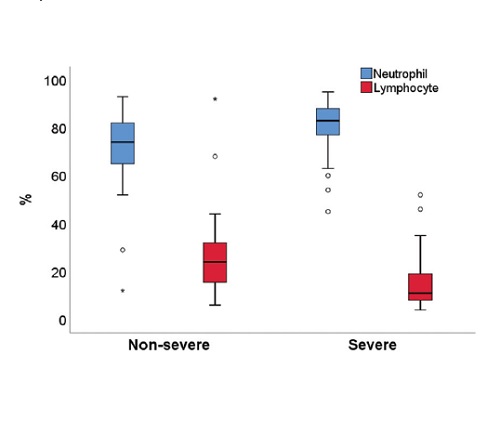
Methodology. In this retrospective analytic study, we identified 325 out of 1,049 adult Filipino inpatients diagnosed with COVID-19 and analyzed their Ct values and pertinent laboratory biomarkers such as neutrophil and lymphocyte count, platelet count, LDH, ferritin, procalcitonin, CRP, AST/SGOT, ALT/SGPT, PT/INR, and D-dimer, and correlated them with the severity of the disease.
Results. Two hundred twenty (67.7%) patients had non-severe disease, while 105 (32.3%) had severe disease. Lower Ct values of ORF1ab (median = 26.4) and N (median = 24.8) genes were seen in the severe group compared to the non-severe group and were found to be significant (p<0.001). Laboratory markers (neutrophil, platelet counts, LDH, ferritin, procalcitonin, CRP, AST, PT/INR, and D-dimer) were associated with severe COVID-19. On the other hand, ALT was not associated with severe disease.
Conclusion. The laboratory biomarkers together with Ct value and overall clinical picture may provide valuable information to physicians for more robust clinical decision-making.
Downloads
References
Ludwig S, Zarbock A. Coronaviruses and SARS-CoV-2: a brief overview. Anesth Analg. 2020;131(1):93-6. https://pubmed.ncbi.nlm.nih.gov/32243297. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7173023. https://doi.org/10.1213/ANE.0000000000004845. DOI: https://doi.org/10.1213/ANE.0000000000004845
World Health Organization. Philippines Coronavirus Disease 2019 (COVID-19 ) Situation Report # 83. Transmission stage assessment; 2021.
Philippine Society for Microbiology and Infectious Diseases. Interim guidance on the clinical management of adult patients with suspected or confirmed COVID-19 infection (version 3.1); 2020. https://www.psmid.org/interim-management-guidelines-for-covid-19-version-3-1/.
World Health Organization. WHO in-house assays for 2019-novel coronavirus (2019-nCoV) real-time rRT-PCR; 2020. https://www.who.int/docs/default-source/coronaviruse/whoinhouseassays.pdf. Accessed February 28, 2022.
Wang H, Li X, Li T, et al. The genetic sequence, origin, and diagnosis of SARS-CoV-2. 2020;39(9):1629-35. https://pubmed.ncbi.nlm.nih.gov/32333222. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7180649. https://doi.org/10.1007/s10096-020-03899-4. DOI: https://doi.org/10.1007/s10096-020-03899-4
McPherson RA, Pincus MR. Henry’s clinical diagnosis and management by laboratory methods. 24th ed. Elsevier Inc.; 2021.
Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. 2020;9(3):573-86. https://pubmed.ncbi.nlm.nih.gov/32725536. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7386165. https://doi.org/10.1007/s40121-020-00324-3. DOI: https://doi.org/10.1007/s40121-020-00324-3
Tom MR, Mina MJ. To interpret the SARS-CoV-2 Test, consider the cycle threshold value. 2020;71(16):2252-4. https://pubmed.ncbi.nlm.nih.gov/32435816. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7314112. https://doi.org/10.1093/cid/ciaa619. DOI: https://doi.org/10.1093/cid/ciaa619
AACC recommendation for reporting SARS-CoV-2 cycle threshold (CT) values. 2021. www.aacc.org/-/media/Files/Science-and-Practice/Statements/Recommendationsreporting-SARSCoV2-CT-values.pdf.
IDSA and AMP joint statement on the use of SARS-CoV-2 PCR cycle threshold (Ct) values for clinical decision-making. 2021. https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-amp-statement.pdf.
APHL. Ct values: what they are and how they can be used. 2020. https://www.aphl.org/programs/preparedness/Crisis-Management/Documents/APHL-COVID19-Ct-Values.pdf.
Punchoo R, Bhoora S, Bangalee A. Laboratory considerations for reporting cycle threshold value in COVID-19. EJIFCC. 2022;33(2):80–93. https://pubmed.ncbi.nlm.nih.gov/36313906. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9562486.
Tsang NNY, So HC, Ng KY, Cowling BJ, Leung GB, Ip DKM. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21(9):1233-45. https://pubmed.ncbi.nlm.nih.gov/33857405. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8041361. https://doi.org/10.1016/S1473-3099(21)00146-8. DOI: https://doi.org/10.1016/S1473-3099(21)00146-8
Moutchia J, Pokharel P, Kerri A, et al. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS One. 2020;15(10):e0239802. https://pubmed.ncbi.nlm.nih.gov/33002041. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7529271. https://doi.org/10.1371/journal.pone.0239802. DOI: https://doi.org/10.1371/journal.pone.0239802
He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–5. https://pubmed.ncbi.nlm.nih.gov/32296168. https://doi.org/10.1038/s41591-020-0869-5. DOI: https://doi.org/10.1038/s41591-020-0869-5
Hall SM, Landaverde L, Gill CJ. Comparison of anterior nares CT values in asymptomatic and symptomatic individuals diagnosed with SARS-CoV-2 in a university screening program. PLoS ONE. 2022;17(7):e0270694. https://pubmed.ncbi.nlm.nih.gov/35830378. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9278773. https://doi.org/10.1371/journal.pone.0270694. DOI: https://doi.org/10.1371/journal.pone.0270694
Long H, Zhao J, Zeng HL, et al. Prolonged viral shedding of SARS-CoV-2 and related factors in symptomatic COVID-19 patients: a prospective study. BMC Infect Dis. 2021;21(1):1282. https://pubmed.ncbi.nlm.nih.gov/34961470. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8711078. https://doi.org/10.1186/s12879-021-07002-w. DOI: https://doi.org/10.1186/s12879-021-07002-w
Yan D, Liu XY, Zhu YN, et al. Factors associated with prolonged viral shedding and impact of lopinavir/ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur Resp J. 2020;56(1): 2000799. https://pubmed.ncbi.nlm.nih.gov/32430428. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7241115. https://doi.org/10.1183/13993003.00799-2020. DOI: https://doi.org/10.1183/13993003.00799-2020
Zhu H, Fu L, Jin Y, et al. Clinical features of COVID-19 convalescent patients with re-positive nucleic acid detection. J Clin Lab Anal. 2020;34(7):e23392. https://pubmed.ncbi.nlm.nih.gov/32506726. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7300578. https://doi.org/10.1002/jcla.23392. DOI: https://doi.org/10.1002/jcla.23392
Samprathi M, Jayashree M. Biomarkers in COVID-19: an up-to-date review. Front Pediatr. 2021;8:607647. https://pubmed.ncbi.nlm.nih.gov/33859967. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8042162. https://doi.org/10.3389/fped.2020.607647. DOI: https://doi.org/10.3389/fped.2020.607647
Ghahramani S, Tabrizi R, Lankarani KB, et al. Laboratory features of severe vs . non ‑ severe COVID ‑ 19 patients in Asian populations : a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):30. https://pubmed.ncbi.nlm.nih.gov/32746929. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7396942. https://doi.org/10.1186/s40001-020-00432-3. DOI: https://doi.org/10.1186/s40001-020-00432-3
Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta. 2020;510:475-82. https://pubmed.ncbi.nlm.nih.gov/32798514. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7426219. https://doi.org/10.1016/j.cca.2020.08.019. DOI: https://doi.org/10.1016/j.cca.2020.08.019
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89-95. https://pubmed.ncbi.nlm.nih.gov/11240971. https://doi.org/10.1067/mcp.2001.113989. DOI: https://doi.org/10.1067/mcp.2001.113989
Sansure Biotech Inc. Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluoresence Probing). 2020. https://www.sansureglobal.com/product/s3102e-sc2/.
Shah VP, Farah WH, Hill JC, et al. Association between SARS-CoV-2 Cycle threshold values and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. Open Forum Infect Dis. 2021;8(9):ofab453. https://pubmed.ncbi.nlm.nih.gov/34584900. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8465328. https://doi.org/10.1093/ofid/ofab453. DOI: https://doi.org/10.1093/ofid/ofab453
Rabaan AA, Tirupathi R, Sule AA, Aldali J, Mutair A Al. Viral dynamics and real-time RT-PCR Ct values correlation with disease severity in COVID-19. Diagnostics (Basel). 2021;11(6):1091. https://pubmed.ncbi.nlm.nih.gov/34203738. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8232180. https://doi.org/10.3390/diagnostics11061091. DOI: https://doi.org/10.3390/diagnostics11061091
Camargo JF, Lin RY, Komanduri K V. Lack of correlation between the SARS-CoV-2 cycle threshold (Ct) value and clinical outcomes in patients with COVID-19. J Med Virol. 2021;93(10):6059-62. https://pubmed.ncbi.nlm.nih.gov/34196409. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8427039. https://doi.org/10.1002/jmv.27171. DOI: https://doi.org/10.1002/jmv.27171
Joynt GM, Wu WK. Understanding COVID-19: what does viral RNA load really mean? Lancet Infect Dis. 2020;20(6):635-6. https://pubmed.ncbi.nlm.nih.gov/32224308. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7118539. https://doi.org/10.1016/S1473-3099(20)30237-1. DOI: https://doi.org/10.1016/S1473-3099(20)30237-1
Ruiz SJ, Ventura PS, García MM, et al. Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. Eur J Clin Invest. 2021;51(1):e13404. https://pubmed.ncbi.nlm.nih.gov/32918295. https://doi.org/10.1111/eci.13404. DOI: https://doi.org/10.1111/eci.13404
Tatum D, Taghavi S, Houghton A, Stover J, Toraih E, Duchesne J. Neutrophil-to-lymphocyte ratio and outcomes in Louisiana COVID-19 patients. Shock. 54)5):652-8. https://pubmed.ncbi.nlm.nih.gov/32554992. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7326320. https://doi.org/10.1097/SHK.0000000000001585. DOI: https://doi.org/10.1097/SHK.0000000000001585
Pablo J, Hinojosa R, Rodriguez Y, et al. Association between cycle threshold ( C t ) values and clinical and laboratory data in inpatients with COVID ‐ 19 and asymptomatic health workers. J Med Virol. 2021;93(10):5969-76. https://pubmed.ncbi.nlm.nih.gov/34196423. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8427125. https://doi.org/10.1002/jmv.27170. DOI: https://doi.org/10.1002/jmv.27170
Martín-Rojas RM, Pérez-Rus G, Delgado-Pinos VE, et al. COVID‐19 coagulopathy: an in‐depth analysis of the coagulation system. Eur J Haematol. 2020;105(6):741-50. https://pubmed.ncbi.nlm.nih.gov/32749010. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7436538. https://doi.org/10.1111/ejh.13501. DOI: https://doi.org/10.1111/ejh.13501
Ichkawa Y, Wada H, Ezaki M, et al. Elevated D-dimer levels predict a poor outcome in critically ill patients. Clin Appl Thromb Hemost. 2020;26:1076029620973084. https://pubmed.ncbi.nlm.nih.gov/33347372. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7755937. https://doi.org/10.1177/1076029620973084. DOI: https://doi.org/10.1177/1076029620973084
Sayit AT, Elmali M, Gedikli O. Relationship between acute phase reactants and prognosis in patients with or without COVID-19 pneumonia. Rev Inst Med Trop Sao Paulo. 2021;63:e51. DOI: https://doi.org/10.1590/s1678-9946202163051
Yormaz B, Ergun D, Tulek B, et al. The evaluation of prognostic value of acute phase reactants in the COVID-19. Bratisl Lek Listy. 2020;121(9):628-33. https://pubmed.ncbi.nlm.nih.gov/32990010. https://doi.org/10.4149/BLL_2020_103. DOI: https://doi.org/10.4149/BLL_2020_103
Vanichkachorn G, Newcomb R, Cowl CT, et al. Post COVID-19 syndrome (Long Haul Syndrome): description of a multidisciplinary clinic at the Mayo Clinic and characteristics of the initial patient cohort. Mayo Clin Proc. 2021;96)7):1782-91. https://pubmed.ncbi.nlm.nih.gov/34218857. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8112396. https://doi.org/10.1016/j.mayocp.2021.04.024. DOI: https://doi.org/10.1016/j.mayocp.2021.04.024
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 PJP

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.








