Proficiency Testing Program for Screening Drug Testing Laboratories in the Philippines, 2009-2019
DOI:
https://doi.org/10.21141/PJP2024.05Keywords:
laboratories, quality control, accreditation, drug testing, methamphetamine, tetrahydrocannabinoL, proficiency testingAbstract
Background. According to the guidelines of the Department of Health (DOH)’s Health Facilities and Services Regulatory Bureau (HFSRB), accreditation of drug testing laboratories (DTLs) requires annual participation in a proficiency testing (PT) program. Since 2009, the National Reference Laboratory for Environmental and Occupational Health, Toxicology and Micronutrient Assay of the East Avenue Medical Center (NRL-EAMC) has conducted the PT program for DTLs.
Objectives. This article aims to provide a general overview of the PT program conducted for screening drug testing laboratories (SDTLs) and to examine data on laboratories’ participation and performance in the PT program.
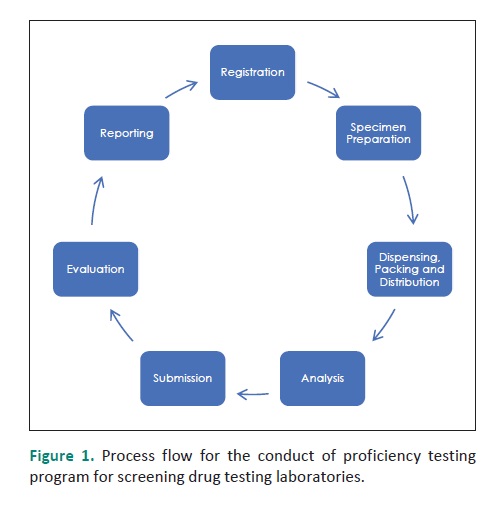
Methodology. Laboratories registered for the PT program were given ten 3-mL synthetic urine specimens which may or may not contain drugs of abuse such as methamphetamine and tetrahydrocannabinol at or above the cut-off level. Laboratories analyzed the PT specimens using immunoassay test kits. The results of the analysis were reported back to NRL-EAMC. The performance of the laboratories in the PT depends on the number of incorrect responses.
Results. For ten years (2009-2019), 1102 ± 188 laboratories annually participated in the program. The mean passing rate was 96.6 ± 4.8%. The number of laboratories which initially failed the PT program significantly decreased from 2009 (15.1%) to 2012 (1.5%). From 2013 to 2019, only below 2.5% of the participating laboratories initially failed the PT. On average, 48.4 ± 18.4% of the laboratories achieved an excellent performance, 34.0 ± 13.6% had a highly satisfactory performance, and 14.3 ± 5.4% got an acceptable performance.
Conclusion. The continued decreasing number of laboratories which failed the PT signifies the improvement of laboratories in urine drug testing. In general, some laboratories participating in the PT for the first time are the ones which initially fail the PT which could be due to a lack of experience in handling PT test items. The PT program highlights the effectiveness of quality control procedures being implemented in a drug testing laboratory.
Downloads
References
Tamama K. Advances in drugs of abuse testing. Clin Chim Acta. 2021; 514:40-7. https://pubmed.ncbi.nlm.nih.gov/33333045. https://doi.org/10.1016/j.cca.2020.12.010. DOI: https://doi.org/10.1016/j.cca.2020.12.010
Lina JD. Board Regulation No. 2 Series of 2003: implementing rules and regulations governing the accreditation of drug testing laboratories in the Philippines. Dangerous Drugs Board. 2003. https://ddb.gov.ph/images/Board_Regulation/2003/Bd.%20Reg.%202%2003.pdf. Accessed September 9, 2023.
Avenido AS. Board Regulation No. 3 Series of 2006: Guidelines for the Drug Proficiency Testing (PT) Program for Drug Testing Laboratories (DTLs). Dangerous Drugs Board. 2006. https://ddb.gov.ph/images/Board_Regulation/2006/Bd.%20Reg.%203%2006.pdf. Accessed September 9, 2023.
Rosell-Ubial PJB. Department Circular No. 2017-0173: agreement on the new timelines for the application and issuance of certificate of performance / certificates of proficiency of External Quality Assessment Program (EQAP) Conducted by National Reference Laboratories (NRLs). Department of Health.
Khan LB, Read HM, Ritchie SR, Proft T. Artificial urine for teaching urinalysis concepts and diagnosis of urinary tract infection in the medical microbiology laboratory. J Microbiol Biol Educ. 2017;18(2):18.2.46. https://pubmed.ncbi.nlm.nih.gov/28861143. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5577974. https://doi.org/10.1128/jmbe.v18i2.1325. DOI: https://doi.org/10.1128/jmbe.v18i2.1325
Shi Z, Dai C, Deng P, et al. Smartphone-based portable photoelectrochemical biosensing system for point-of-care detection of urine creatinine and albumin. Lab Chip. 2023;23(15):3424-3432. https://pubmed.ncbi.nlm.nih.gov/37404057. https://doi.org/10.1039/D3LC00238A. DOI: https://doi.org/10.1039/D3LC00238A
Dangerous Drugs Board. Profile of Drug Abusers (Facility Based) CY 2022. Dangerous Drugs Board. https://ddb.gov.ph/2022-statistical-analysis/#:~:text=About%20twenty%2Dfive%20percent%20(24.53,15%20to%2019%20years%20old. Accessed September 9, 2023.
Ríos A, Barceló D, Buydens L, et al. Quality assurance of qualitative analysis in the framework of the European project ’MEQUALAN’. Accreditation and Quality Assurance. 2003;8(2):68-77. https://doi.org/10.1007/s00769-002-0556-x. DOI: https://doi.org/10.1007/s00769-002-0556-x
Abaya JEA, Roxas MA, Ona ET. Implementing rules and regulations Republic Act 10586 “Anti- Drunk and Drugged Driving Act of 2013.” DOTC, DILG, and DOH. 2014. https://www.officialgazette.gov.ph/downloads/2014/04apr/20140428-IRR-RA-10586.pdf. Accessed September 16, 2023.
Ochoa PN. Memorandum Circular No. 89: Implementation and Institutionalization of the National Anti-Drug Plan of Action. Office of the President. 2015. https://www.officialgazette.gov.ph/downloads/2015/12dec/20151217-MC-0089-BSA.pdf. Accessed September 10, 2023.
Simbulan N, Estacio L, Dioquino-Maligaso C, Herbosa T, Withers M. The Manila declaration on the drug problem in the Philippines. Ann Glob Health. 2019;85(1):26. https://pubmed.ncbi.nlm.nih.gov/30873786. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6634291. https://doi.org/10.5334/aogh.28. DOI: https://doi.org/10.5334/aogh.28
dela Rosa- Bala A. Memorandum Circular No. 13 s. 2017 Guidelines in the Mandatory Random Drug Test for Public Officials and Employees and for other Purposes. Civil Service Commission. 2017. https://oshc.dole.gov.ph/wp-content/uploads/2021/05/CSC-MC-13-s-2017.pdf. Accessed September 18, 2023.
Crisostomo S. DOLE reminds employers to conduct random drug tests. The Philippine Star. October 23, 2016. https://www.philstar.com/headlines/2016/10/23/1635981/dole-reminds-employers-conduct-random-drug-tests. Accessed September 13, 2023.
Segura J, de la Torre R, Congost M, Camí J. Proficiency testing on drugs of abuse: one year’s experience in Spain. Clin Chem. 1989;35(5):879-83. https://pubmed.ncbi.nlm.nih.gov/2720987. DOI: https://doi.org/10.1093/clinchem/35.5.879
Wilson JF, Smith BL, Toseland PA, et al. External Quality Assessment of Techniques for the detection of drugs of abuse in urine. Ann Clin Bioch. 1994;31(Pt 4):335-42. https://pubmed.ncbi.nlm.nih.gov/7979098. https://doi.org/10.1177/000456329403100405. DOI: https://doi.org/10.1177/000456329403100405
Guerrant GO, Hall CT. Drug abuse proficiency testing. Clin Toxicol. 1977;10(2):209-19. Phttps://pubmed.ncbi.nlm.nih.gov/852242. https://doi.org/10.3109/15563657708987967. DOI: https://doi.org/10.3109/15563657708987967
Frings CS, White RM, Battaglia DJ. Status of drugs-of-abuse testing in urine: an AACC study. Clin Chem. 1987;33(9):1683-6. Phttps://pubmed.ncbi.nlm.nih.gov/3621586. https://doi.org/10.1093/clinchem/33.9.1683. DOI: https://doi.org/10.1093/clinchem/33.9.1683
Ferrara SD, Brusini G, Maietti S, et al. Proficiency testing for psychoactive substances in Italy. Int J Legal Med. 1999;113(1):50-4. https://pubmed.ncbi.nlm.nih.gov/10654240. https://doi.org/10.1007/s004140050279. DOI: https://doi.org/10.1007/s004140050279
United Nations Office on Drugs and Crime. International Collaborative Exercises (ICE). https://www.unodc.org/unodc/en/scientists/ice_new.html. Accessed October 28, 2023.
Krasowski MD, McMillin GA, Melanson SEF, Dizon A, Magnani B, Snozek CLH. Interpretation and utility of drug of abuse screening immunoassays: insights from laboratory drug testing proficiency surveys. Arch Pathol Lab Med. 2020;144(2):177-84. https://pubmed.ncbi.nlm.nih.gov/31313960. https://doi.org/10.5858/arpa.2018-0562-CP. DOI: https://doi.org/10.5858/arpa.2018-0562-CP
Bordeerat NK, Fongsupa S, Dansethakul P, Rungpanitch U, Pidetcha P. Establishing an External Quality Assessment (EQA) Program for urinalysis in medical laboratories of Thailand. Ind J Clin Biochem. 2024;39(2):271-5. https://pubmed.ncbi.nlm.nih.gov/38577144. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10987422 (available on 2025-04-01). https://doi.org/10.1007/s12291-022-01102-3. DOI: https://doi.org/10.1007/s12291-022-01102-3
Sarigul N, Korkmaz F, Kurultak İ. A new artificial urine protocol to better imitate human urine. Sci Rep. 2019;9(1):20159. https://pubmed.ncbi.nlm.nih.gov/31882896. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6934465. https://doi.org/10.1038/s41598-019-56693-4. DOI: https://doi.org/10.1038/s41598-019-56693-4
Beavis G, Wilson J, Sykes M. Quantitative scores for binary qualitative proficiency testing. Accreditation and Quality Assurance. 2019;24(4):263-9. DOI: https://doi.org/10.1007/s00769-019-01386-8
International Organization for Standardization. ISO 17043:2023 Conformity assessment- General requirements for the competence of proficiency testing providers. https://www.iso.org/standard/80864.html.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 PJP

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

The Philippine Journal of Pathology is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Based on works made open access at http://philippinejournalofpathology.org








