Spit or Swab? The Diagnostic Accuracy of Saliva-Based Testing as a Sars-Cov-2 Surveillance Tool
DOI:
https://doi.org/10.21141/PJP.2022.14Keywords:
qRT-PCR, rapid antigen test, pooling, SARS-CoV-2, COVID-19Abstract
Background. Nasopharyngeal swab/oropharyngeal swab (NPS/OPS) qRT-PCR is the gold standard for detecting SARS-CoV-2. However, it has its own limitations including cost and invasiveness. As an alternative, individual qRT-PCR testing of saliva samples was validated and shown to be comparable in sensitivity and specificity with NP-OP qRT-PCR. To further maximize its utility, the researchers wish to explore antigen and pooled testing methods.
Objective. The study aimed to evaluate the diagnostic accuracy of detecting SARS-CoV-2 infection using saliva-based pooled qRT-PCR and rapid antigen test compared with individual saliva qRT-PCR.
Methodology. In this retrospective cross-sectional study, saliva specimen from individuals aged 18 years old and above from the outpatient specimen collection station at the Philippine Children’s Medical Center were tested individually using qRT-PCR (Mag-bind RNA Extraction Kit/MACURA, Allsheng Extraction Machine, Sansure PCR kit, and MA-600 Sansure Biotech). Non-probability convenience sampling was utilized. Based on the individual results, pools of five (5) individual specimens, which includes one (1) positive sample were tested with qRT-PCR for sensitivity. DNK-2150-1S Dynamiker SARS-CoV-2 Ag Rapid Test (Dynamiker Biotechnology Co., Ltd., Tianjin, China) was also used to test individual saliva specimens.
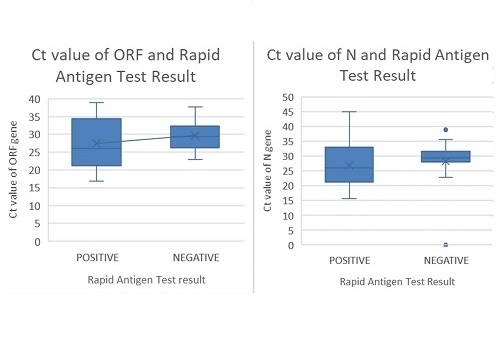
. Out of 196 individual saliva specimens, 73 were detected to have SARS-COV-2 by qRT-PCR, while the remaining 123 were negative. Compared with the individual saliva qRT-PCR, rapid antigen tests done showed sensitivity of 46.58% (95% CI 35.13%, 58.02%), specificity of 86.18% (95% CI 80.08%, 92.28%), positive and negative predictive value of 66.67% (95% CI 53.71%, 79.60%) and 73.10% (95% CI 65.89%, 80.32%) respectively. Based on the results of individual saliva-based qRT-PCR, 62 pools were tested and showed sensitivity of 98.39% (95% CI 91.34%, 99.96%).
Conclusion and Recommendation. Pooled saliva-based testing for SARS-CoV-2 is comparable with individual saliva-based rapid antigen testing. The use of rapid antigen testing is less sensitive and less specific compared with qRT-PCR consistent with prior reports. Additional studies are recommended to determine optimal conditions for testing.
Downloads
References
2. Lo R, Barrientos A, Espiritu B, Santiago FK, et al. An Evaluation of pooling strategies for RT-qPCR testing for SARS-CoV-2 infection: a pragmatic multi-site parallel operational study. Philipp J Pathol. 2020;5(2):12–33. https://doi.org/10.21141/PJP.2020.12.
3. Cerutti F, Burdino E, Milia MG, et al. Urgent need of rapid tests for SARS CoV-2 antigen detection: evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J Clin Virol. 2020;132:104654. https://pubmed.ncbi.nlm.nih.gov/3305349. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7522649. https://doi.org/10.1016/j.jcv.2020.104654.
4. Duque FT. Interim guidelines for the conduct of saliva-based RT-PCR testing for the detection of SARS CoV-2; 2021. Department of Health. https://doh.gov.ph/sites/default/files/health-update/dm2021-0161.pdf. Accessed February 14, 2021.
5. Fogarty A, Joseph A, Shaw D. Pooled saliva samples for COVID-19 surveillance programme. Lancet Respir Med. 2020;8(11):1078–80. https://pubmed.ncbi.nlm.nih.gov/32976755. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7508511. https://doi.org/10.1016/S2213-2600(20)30444-6.
6. Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity - a strategy for containment. N Engl J Med. 2020;383(22):e120. https://pubmed.ncbi.nlm.nih.gov/32997903. https://doi.org/10.1056/NEJMp2025631.
7. Ranoa DRE, Holland RL, Alnaji FG, et al. Saliva-based molecular testing for SARS-CoV-2 that Bypasses RNA extraction. bioRxiv. https://doi.org/10.1101/2020.06.18.159434.
8. To KK-W, Tsang OT-Y, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–74. https://pubmed.ncbi.nlm.nih.gov/32213337. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7158907. https://doi.org/10.1016/S1473-3099(20)30196-1.
9. Wyllie AL, Fournier J, Casanovas-Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383(13):1283–6. https://pubmed.ncbi.nlm.nih.gov/32857487. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7484747. https://doi.org/10.1056/NEJMc2016359.
10. Watkins AE, Fenichel EP, Weinberger DM, et al. Increased SARAS-CoV-2 testing capacity with pooled saliva samples. Emerg Infect Dis. 2021;27(4):1184-7. https://pubmed.ncbi.nlm.nih.gov/33755009. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8007323. https://doi.org/10.3201/eid2704.204200.
11. Nagura-Ikeda M, Imai K, Tabata S, et al. Clinical evaluation of self-collected saliva by quantitative reverse transcription-PCR (RT-qPCR), direct RT-qPCR, reverse transcription–loop-mediated isothermal amplification, and a rapid antigen test to diagnose COVID-19. J Clin Microbiol. 2020;58(9) :e01438-20. https://pubmed.ncbi.nlm.nih.gov/32636214. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7448663. https://doi.org/10.1128/JCM.01438-20.
12. Barat B, Das S, De Giorgi V, et al. Pooled saliva specimens for SARS-CoV-2 testing. J Clin Microbiol. 2021;59(3): e02486-20. https://pubmed.ncbi.nlm.nih.gov/33262219. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8106731. https://doi.org/10.1128/JCM.02486-20.
13. Rainey A, Pierce A, Deng X, et al. Validation and deployment of a direct saliva real-time RT-PCR test on pooled samples for COVID-19 surveillance testing. PLoS One. 2021;16(12):e0261956. https://pubmed.ncbi.nlm.nih.gov/34969053. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8718011. https://doi.org/10.1371/journal.pone.0261956.
14. Dynamiker Biotechnology (Tianjin) Co. L. DNK-2105-1S Dynamiker SARS-CoV-2. http://en.dynamiker.com/index/index/pro_info/aid/640.html. Accessed October 20, 2021.
15. Dynamiker SARS-CoV-2 AG rapid test (saliva). FIND Diagnosis for all. https://www.finddx.org/test-directory/?subpage=variants-panel. Accessed October 20, 2021.
16. Diao B, Wen K, Chen J, et al. Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. medRxiv. https://doi.org/10.1101/2020.03.07.20032524. Accessed January 1, 2020.
17. De Marinis Y, Pesola A-K, Söderlund Strand A, Norman A, Pernow G, Aldén M, et al. Detection of SARS-CoV-2 by rapid antigen tests on saliva in hospitalized patients with COVID-19. Infect Ecol Epidemiol. 2021;11(1):1993535. https://pubmed.ncbi.nlm.nih.gov/34745449. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8567870. https://doi.org/10.1080/20008686.2021.1993535.
18. Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform. 2014;48:193–204. https://pubmed.ncbi.nlm.nih.gov/24582925. https://doi.org/10.1016/j.jbi.2014.02.013.
19. Department of Health Philippines. Department of Health - nCOV tracker; 2020. https://ncovtracker.doh.gov.ph/. Accessed September 18, 2020.
20. Oguri S, Fujisawa S, Kamada K, et al. Effect of varying storage conditions on diagnostic test outcomes of SARS-CoV-2. J Infect. 2021;83(1):119–45. https://pubmed.ncbi.nlm.nih.gov/33823203. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8018904. https://doi.org/10.1016/j.jinf.2021.03.026.
21. Buban JMA, Villanueva PN, Gregorio GE. Should RT-PCR of saliva samples be used for diagnosis of COVID19? Philippine COVID-19 Living Clinical Practice Guidelines Inst Clin Epidemiol Natl Institutes Heal UP Manila; 2021. Available from https://www.psmid.org/wp-content/uploads/2021/05/SALIVA-RT-PCR-CPG-FINAL_031521_MMA.pdf.
22. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for Detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-60. https://pubmed.ncbi.nlm.nih.gov/33449069. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7811189. https://doi.org/10.1001/jamainternmed.2020.8876.
23. The Health Technology Assessment Council (HTAC). HTAC recommendation on the use of saliva as an alternative specimen for RT-PCR testing; 2021. Available from https://hta.doh.gov.ph/wp-content/uploads/2021/05/Evidence_Summary_RT-PCR_Testing_for_COVID-19-Recommendation.pdf.
24. Product evaluation status antigen test kits. Research Institute for Tropical Medicine. https://ritm.gov.ph/covid-19-kit-evaluation/ongoing-evaluations/product-evaluation-status-antigen-test-kits/. Accessed January 29, 2022.
25. Seitz T, Schindler S, Winkelmeyer P, et al. Evaluation of rapid antigen tests based on saliva for the detection of SARS‐CoV‐2. J Med Virol. 2021;93(7):4161–2. https://pubmed.ncbi.nlm.nih.gov/33788280. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8251355. https://doi.org.10.1002/jmv.26983.
26. Potential for false positive results with antigen tests for rapid detection of SARS-CoV-2 - letter to clinical laboratory staff and health care providers. USFDA. https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-positive-results-antigen-tests-rapid-detection-sars-cov-2-letter-clinical-laboratory. Accessed January 29, 2022.
27. Gonong DA, Misiona G, Sionzon M, Santiago FK, Lira AJ, Lo R. Analysis of SARS-CoV-2 RT-PCR testing and pooling strategies for screening of asymptomatic individuals - the Philippine Children’s Medical Center experience. Philipp J Pathol. 2021;6(1):18–25. https://doi.org/10.21141/PJP.2021.03.
28. Sahajpal NS, Mondal AK, Ananth S, et al. SalivaSTAT: direct-PCR and pooling of saliva samples collected in healthcare and community setting for SARS-CoV-2 mass surveillance. Diagnostics (Basel). 2021;11(5):904. https://pubmed.ncbi.nlm.nih.gov/34069462. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8159081. https://doi.org/10.3390/diagnostics11050904.
29. Esteves E, Mendes AK, Barros M, et al. Population wide testing pooling strategy for SARS-CoV-2 detection using saliva. PLoS One. 2022;17(1):e0263033. https://pubmed.ncbi.nlm.nih.gov/35089942. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8797214. https://doi.org/10.1371/journal.pone.0263033.
30. Padua Jr. R. Diagnostic testing strategies to manage COVID-19 pandemic: proposed by the Philippine Society of Pathologists, Inc. Philipp J Pathol. 2020;5(1):5–8. https://doi.org/10.21141/PJP.2020.08.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 PJP

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

The Philippine Journal of Pathology is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Based on works made open access at http://philippinejournalofpathology.org








