Evaluation of Rapid Antigen Testing (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 Diagnosis in a Tertiary Hospital
DOI:
https://doi.org/10.21141/PJP2024.07Keywords:
COVID-19, Antigen Testing, CT-value, LFA, RT-PCR, SARS-CoV-2Abstract
Background. The Panbio™ COVID-19 Ag Rapid Test is a Food and Drug Administration (FDA)-approved point-of-care test (POCT) used for SARS-CoV-2 detection which has met minimum sensitivity and specificity requirements by the World Health Organization (WHO).
Objective. The study aimed to compare the clinical performance of a commercial lateral flow assay (LFA) to reverse transcriptase polymerase reaction (RT-PCR) in SARS-CoV-2 infection diagnosis
Methodology. Clinical data and simultaneous LFA and RT-PCR samples collected from June 2021 to June 2022 were obtained to analyze the diagnostic accuracy of LFA compared to RT-PCR.
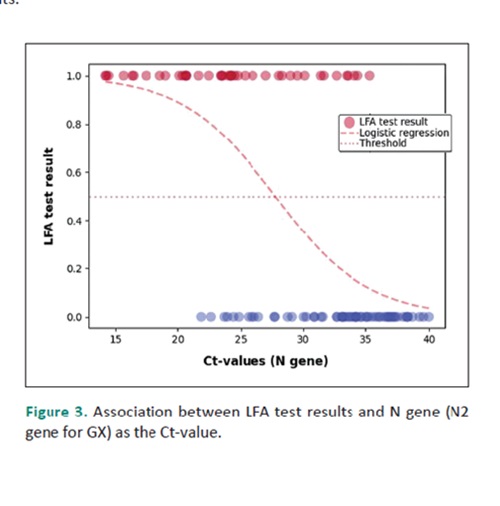
Results. A total of 265 samples was obtained. 34.45% of RT-PCR positive samples were reliably detected by LFA. COVID-19 was reliably ruled out by LFA in 99.32% RT-PCR negative samples. LFA sensitivity among symptomatic patients with ≤7 days of illness was 51.61%, slightly higher than those with >7 days of illness (18.92%), and significantly higher than asymptomatic patients (16.67%). Asymptomatic subjects have a varied range of Ct-values, indicating different stages of infection or viral loads. Individuals with symptoms for more than 7 days have higher Ct-values, suggesting they are in later stages of infection or have lower viral loads. The probability of a positive LFA result decreases significantly when the Ct-value is beyond 28-30.
Conclusion. The LFA evaluated in this study did not show significant sensitivity and specificity during the early disease course wherein viral loads are suggestively high. However, its utility to accurately rule out COVID-19 is quite reliable in subjects with symptoms that are >7 days since Ct-values are suggestively beyond 28-30 which implies a significantly decreased probability of a positive LFA result.
Downloads
References
Centers of Disease Control and Prevention. Overview of testing for SARS-CoV-2 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. DOI: https://doi.org/10.46234/ccdcw2020.085
Health Technology Assessment Council and Health Technology Assessment Unit. Evidence summary: use of rapid antigen test kits for the diagnosis of COVID-19. October 2020. https://hta.dost.gov.ph/wp-content/uploads/2020/10/Annex-C_Updated-Evidence-Summary-on-COVID-19-Rapid-Antigen-Tests-02-October-2020.pdf.
World Health Organization. Antigen detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays: interim guidance. 11 September 2020. https://iris.who.int/handle/10665/334253.
Centers of Disease Control and Prevention. Antigen testing for SARS-CoV-2 (COVID-19). Updated May 2023. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html.
He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672−5. https://pubmed.ncbi.nlm.nih.gov/32296168. https://doi.org/10.1038/s41591-020-0869-5. DOI: https://doi.org/10.1038/s41591-020-0869-5
Engelmann I, Alidjinou EA, Ogiez J, et al. preanalytical issues and cycle threshold values in SARS-CoV-2 Real-Time RT-PCR testing: should test results include these? ACS Omega 2021;6(10):6528-36. https://pubmed.ncbi.nlm.nih.gov/33748564. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7970463. https://doi.org/10.1021/acsomega.1c00166. DOI: https://doi.org/10.1021/acsomega.1c00166
Linares M, Pérez-Tanoira R, Carrero A, et al. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J Clin Virol. 2020;133:104659. https://pubmed.ncbi.nlm.nih.gov/33160179. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7561603. https://doi.org/10.1016/j.jcv.2020.104659. DOI: https://doi.org/10.1016/j.jcv.2020.104659
Mboumba Bouassa RS, Veyer D, Péré H, Bélec L. Analytical performances of the point-of-care SIENNATM COVID-19 Antigen Rapid Test for the detection of SARS-CoV-2 nucleocapsid protein in nasopharyngeal swabs: A prospective evaluation during the COVID-19 second wave in France. Int J Infect Dis. 2021;106:8-12. https://pubmed.ncbi.nlm.nih.gov/33746093. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7970753. https://doi.org/10.1016/j.ijid.2021.03.051. DOI: https://doi.org/10.1016/j.ijid.2021.03.051
Gremmels H, Winkel BMF, Schuurman R, et al. Real-life validation of the Panbio™ COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. ECLinicalMedicine. 2021;31:100677. https://pubmed.ncbi.nlm.nih.gov/33521610. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7832943. https://doi.org/10.1016/j.eclinm.2020.100677. DOI: https://doi.org/10.1016/j.eclinm.2020.100677
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 PJP

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

The Philippine Journal of Pathology is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Based on works made open access at http://philippinejournalofpathology.org








