We report a rare case of prostatic adenocarcinoma with diffuse aberrant p63 expression in the luminal cells. p63-positive prostatic adenocarcinoma often has distinctive morphology and immunoprofile, but may be confused with benign mimickers of prostate cancer. It is suggested that this tumor variant is molecularly distinct from usual type prostatic adenocarcinoma. Despite sometimes exhibiting seemingly unfavorable Gleason patterns, a less aggressive biologic behavior is often observed. Literature regarding molecular profile, morphologic characteristics, grading, and prognosis of this entity is reviewed.
Key words: aberrant, AMACR/HMWCK/p63 cocktail, needle biopsy, prostatic adenocarcinoma, p63
In prostate biopsies, the diagnosis of prostatic adenocarcinoma (PCa) is often challenging, especially when the morphologic features are insufficient to establish a definite diagnosis. In evaluating these equivocal cases, either high-molecular weight cytokeratin (HMWCK) or p63 preferably or a combination of these two with AMACR/p504S/RACEMASE, either in a double or triple cocktail, is commonly used as an adjunctive tool in distinguishing PCa from benign mimickers. The absence of immunostaining for basal cells with the use of p63 and HMWCK, which are nuclear and cytoplasmic antibodies, respectively confirms PCa.[1] In addition, this diagnosis is supported by positive immunostaining for α-methylacyl-CoA racemase (AMACR), a luminal marker that is significantly (but not exclusively) upregulated in PCa.[2]
Recently, cases of an extremely rare variant of PCa with aberrant p63 expression (p63-PCa) have been described. These are characterized by distinct nuclear p63 expression in a non-basal distribution and lack of staining for HMWCK. In 2008, Osunkoya et al., reported a series of 21 cases of p63-PCa on needle biopsy and radical prostatectomy specimens.[3] Since then, a few cases of p63-PCa have been reported and a review of the corresponding radical prostatectomy specimens of the 21 needle biopsies with p63-pCA have been published.[4],[5],[6],[7] Thus far, no such case has been documented in the country. Herein, we present an additional case of p63-PCa and review the existing literature regarding its molecular profile, histomorphologic characteristics, grading and prognostic implications.
We report a case of a 62-year old male who presented with urinary tract infection. Work-up revealed an enlarged prostate gland weighing 25 grams by ultrasonography; and an elevated prostate-specific antigen (PSA) of 13 ng/mL. He underwent a transrectal ultrasound-guided (TRUS) prostate biopsy reported as benign prostatic hyperplasia with high-grade prostatic intraepithelial neoplasia in another institution; hence, he was advised PSA monitoring. A month after the procedure, his PSA decreased to 3.7 ng/mL. However, the patient was lost to follow-up. On consult few months later, PSA was noted to have increased to 6.08 ng/mL. He underwent another TRUS biopsy (now assessed by the primary author), which revealed a small focus of atypical glandspresent suspicious for prostatic adenocarcinoma, in one core. These few well-formed, individual, atypical glands were seen seemingly infiltrating in between benign acini. The glands of interest show rigid lumina and multilayered neoplastic cells which have enlarged hyperchromatic nuclei with rare prominent nucleoli and amphophilic cytoplasm. Immunohistochemical analysis for p63 (antibody clone 7JUL, Biocare Medical®), high molecular weight cytokeratin (HMWCK, antibody clone 34βE12, Leica Bond®), and α-methyl acyl coenzyme-A racemase (AMACR, antibody clone 13H4, Dako®) in a PIN4 cocktail was performed in accordance with the recommendation by International Society of Urological Pathology (ISUP).[8] The atypical glands showed granular luminal and cytoplasmic positivity for AMACR; and no HMWCK-expressing basal cells were identified among these glands. Surprisingly, some of their nuclei were strongly positive for p63 (Figure 1). The final diagnosis was prostatic adenocarcinoma, involving 5% of one core, with diffuse aberrant staining for p63. A Gleason score of 3+3=6 would be designated if this was a classical acinar prostatic adenocarcinoma.
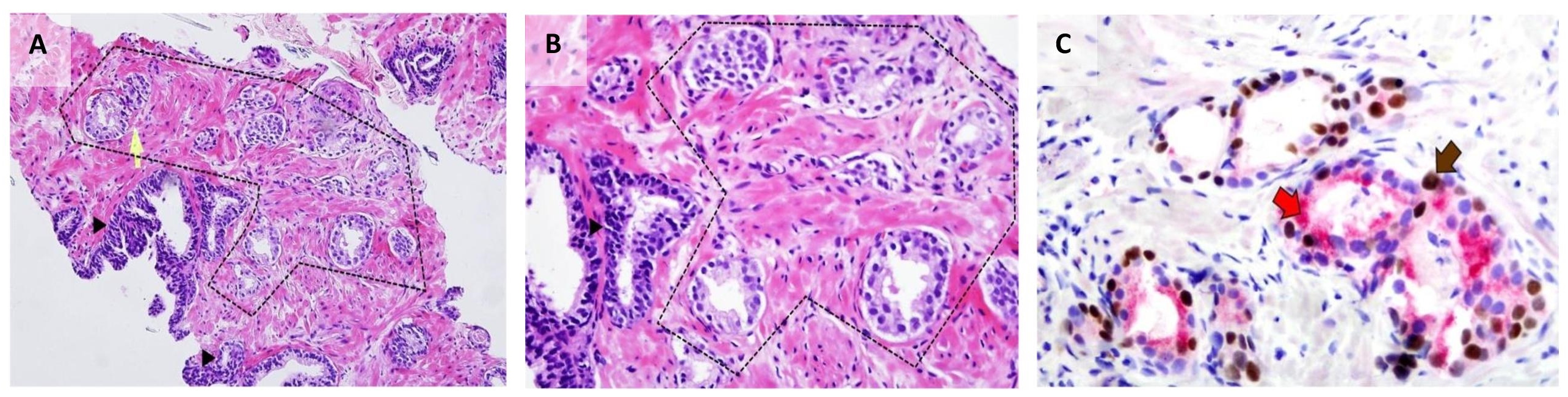
Figure 1. Prostate core biopsy from the study case. (A)Atypical glands (within dashed lines) with rigid luminal borders are seen infiltrating between benign acini (arrowhead) (H&E 100x). (B) On higher magnification, the cells have enlarged hyperchromatic nuclei with prominent nucleoli and moderate amount of amphophilic cytoplasm (H&E 400x). (C) Immunohistochemical staining for PIN4 decorates secretory luminal cells with nuclear and cytoplasmic immunoreactivity for p63 (brown arrow) and AMACR (red arrow), respectively, and lack of basal staining for HMWCK (PIN4 400x).
The role of p63 in prostate development and tumorigenesis
The significance of p63 expression in p63-pCa remains relatively unknown. The transcription factor p63 is encoded by the TP63 locus, which is a member of the TP53 and TP73 family. It plays a critical role in the formation and maintenance of the prostate stems cells that subsequently differentiate into the basal and secretory cells of the mature prostate epithelium.[9] Within normal prostate epithelium, p63 is selectively expressed in the nuclei of basal cells and is consistently absent in the luminal secretory and neuroendocrine cells.[10]
The vast majority of prostate cancers show loss of p63. The role of TP63 in the development of prostate cancer remains controversial. The debate on whether TP63 is a tumor suppressor gene or an oncogene is mostly due to its structural complexity. It contains 16 different exons coding multiple mRNA isoforms that share a common core DNA binding domain but exhibit varying 5’ and 3’ ends. Alternate promoters generate two different N-terminal variants: isoforms with an acidic transactivation domain, which are known as TA isoforms; and isoforms that lack this amino-terminal domain known as ΔN isoforms. Alternative splicing at the 3' end produces three different C-terminal variants, termed α, β, and γ.[11] The predominant isoform in normal prostate and p63-expressing prostatic adenocarcinoma is ΔNp63.[12],[13]
Studies on ΔNp63 expression in p63-PCa, which is demonstrated by ΔNp63-specific polyclonal antibody (p40), show that most of the aberrant p63-positive tumors have diffuse positivity for p40 in 96% of cases (23/24). All conventional PCa were negative for p40 in the tumor cells.[14] Since ΔNp63 acts as an oncogene, the persistence of p63 expression may serve to drive tumorigenesis and allow maintenance of cancer stem cells in p63-PCa.
TP63 mutational analysis and mRNA quantification in human prostate cancer specimens by Takahashi et al., detected no TP63 mutation. However, there is downregulation of p63 expression compared to normal in 39% of cases, and upregulation in 34%of cases.[15] These findings have been challenged since the prostate cancer cell lines examined were generally p63-negative; and contamination with normal basal cells may have confounded the study. To improve purity of the prostate cancer cells used, a similar study was conducted using laser capture microdissection, RT-PCR and gene sequencing for mutational analysis of TP63 in primary tumors, 20 metastases, 28 tumor xenografts, and 7 prostate cancer cell lines. Results showed that the pattern of TP63 mRNA expression in normal prostate tissue is retained in primary prostate cancers, although the levels of expression were markedly reduced.[12] Because similar levels of TP63 mRNA for all isoforms were detected even after laser capture microdissection, prostate cancers undeniably express TP63 mRNA. A potentially functional TP63 mutation was identified in only one prostate tumor. Since majority of the prostate cancer cell lines and patient tumors examined did not contain TP63 mutations, it is suggested that somatic mutations are not the cause of downregulation of p63 expression in majority of prostate cancers. Further, if TP63 is functioning as prostate cancer gene it likely functions as a tumor suppressor.[12] These findings support that it is indeed possible to have prostate cancers with positive p63 immunostaining since the p63 protein is still expressed, albeit in reduced amounts; and may even be upregulated in some cases. The molecular mechanisms underlying the absence of immunostaining of p63 in the vast majority of prostate cancer specimens are yet to be elucidated.
Molecular profile and immunophenotype of p63-PCa
Extensive genomic analyses of prostate cancer have identified copy number alterations, epigenetic perturbations, and chromosomal rearrangements associated with prostate carcinogenesis. Only a few studies have investigated the molecular distinction between p63-PCa and usual-type prostatic adenocarcinomas. Fusions between the androgen-regulated genes, most commonly the androgen-regulated gene transmembrane protease, serine 2 (TMPRSS2) and v-ets erythroblastosis virus E26 oncogene homolog (ERG), occur in approximately 50% of prostate cancers.[16] These rearrangements are highly specific for PCa or high-grade prostatic intraepithelial neoplasia.[17] In a study by Baydar et al., and Wu et al., fluorescence in-situ hybridization on one p63-pCa case and ERG immunohistochemistry on two p63-pCa cases, respectively, were performed; and they found that it lacked TMPRSS2-ERG translocation.[4],[5]
Tan et al., collected 37 p63-PCa tumors on radical prostatectomy and biopsy to characterize p63-PCa based on common molecular changes seen in usual-type prostatic carcinomas, including ERG gene rearrangements, serine protease inhibitor Kazal-type 1 (SPINK1) expression, phosphatase and tensin homolog (PTEN) loss and glutathione S-transferase pi 1 (GSTP1) methylation. Results showed that p63-expressing tumors lacked ERG rearrangements by fluorescence in situ hybridization (0/14) and ERG protein expression (0/37). The lack of ERG protein expression in p63-PCa was highly statistically significant compared to unselected cohorts of usual-type adenocarcinomas at their institution where 49.3% (534/1083) are positive for ERG protein.[13]
A recent study, which employed a bioinformatics approach termed Cancer Outlier Profile Analysis, has suggested that the lack of TMPRSS2-ETS family gene rearrangements in usual-type prostatic adenocarcinoma may be associated with other characteristic molecular changes, such as SPINK1 overexpression. [17] Inactivation of PTEN, a key tumor suppressor gene that is commonly lost in prostate cancer, is strongly associated with ERG fusion-positive tumors.[18] Conversely, in p63-pCa, no tumor expressed SPINK1 or showed PTEN protein loss (0/19).[13]
Hypermethylation of the CpG island at the promoter of GSTP1 has been described as one of the earliest and most commonly found genome alterations arising during prostate carcinogenesis, present in >90% of prostate cancer cases but not in normal prostate tissues. In approximately 95% of usual-type prostatic adenocarcinomas (88/91) cytidine nucleotides in GSTPI promoter sequences of GSTP1 are hypermethylated, resulting in transcriptional silencing of the gene.[19] In contrast, 74% (14/19) of p63-expressing tumors expressed GSTP1 protein, at least focally, and 33% (2/6) entirely lacked GSTP1 CpG island hypermethylation by bisulfite sequencing.[13] Based on these evidences, it appears that p63-positive PCas may represent a molecularly distinct subtype of PCa.
Usual-type prostatic adenocarcinoma exhibit a luminal cell immunophenotype as these tumors lack basal markers, such as p63 and HMWCK, and diffusely express low molecular weight cytokeratins and markers of androgen axis signaling. A study on p63-positive tumors on radical prostatectomy and biopsy evaluated subsets based on their expression of basal and luminal cell markers. Despite p63 positivity, basal cytokeratins such as CK14 and CK15 were negative in all cases (0/8) and CK5/6 was weakly and focally positive in 36% (4/11) of cases. In contrast, these tumors uniformly expressed luminal-type cytokeratin proteins, such as CK18 (13/13) and CK8 (8/8), and markers of androgen axis signaling commonly observed in luminal cells, including androgen receptor (10/11) and NKX3.1 (8/8). These findings demonstrate that p63-pCa have mixed luminal/basal immunoprofile.[13]
Histomorphologic characteristics of p63-PCa
In a study by Osunkoya et al.,[3] 90.5% of cases showed a distinctive morphology composed predominantly of glands, nests, and cords with atrophic cytoplasm, hyperchromatic nuclei, and visible nucleoli. In approximately 16% of cases, usual-type prostatic adenocarcinoma and high-grade prostatic intraepithelial neoplasia were present.[3] Giannico et al., investigated the morphologic features of p63-pCa in 21 radical prostatectomy specimens. In 18 cases (85.7%), p63-PCa showed a distinctive morphology consisting of atrophic, poorly formed glands, with multilayered and often spindled, and basaloid nuclei with prominent nucleoli. In a minority of cases, p63-pCa resembled usual-type acinar atrophic adenocarcinoma or was lined by columnar cells. Similar to usual PCa, the glands exhibit an infiltrative architecture and nuclear atypia including hyperchromatic nuclei and few prominent nucleoli.[7] The current case shows a minute focus of atypical glands (less than a millimeter in dimension) demonstrating the same infiltrative architecture we commonly associate with classical prostatic adenocarcinoma. This was the first feature that alerted our attention that these could be neoplastic glands. However, a departure from the commonly seen atrophic appearance of these glands is that the tumor cells possess more cytoplasmic volume.
Gleason grading was 3+5=8 (38%) and 3+3=6 (28.5%) in majority of the cases. p63-PCa often coexisted with usual-type acinar prostate carcinoma in 85.7% of cases; but these were usually present in separate nodules. Overall, p63-pCa comprised 65% of the total cancer volume.[7] In two other case reports of p63-pCa diagnosed by transrectal ultrasound-guided prostate biopsies, the atypical prostatic glands exhibited an infiltrative pattern. The cells have mildly enlarged nuclei and rare prominent nucleoli. Both cases were graded as Gleason score 3+3=6.[4],[5]
Aberrant p63 expression is a potential diagnostic pitfall because the immunohistochemical profile may be mistaken as that of benign or atypical glands when either only p63 or basal cell cocktails (p63/HMWCK) are used. Giannico et al., found that the acini in p63-pCa appear frequently atrophic, with a high nuclear-cytoplasmic ratio and a basaloid appearance. This distinctive morphology warrants consideration of basal cell proliferations, such as basal cell hyperplasia and basal cell carcinoma.[7] However, the diagnosis of p63-pCa over basal cell proliferation is favored by the lack of HMWCK expression, and positivity of AMACR and PSA.[5]
Prognostic implications
Based on available studies, p63-pCa portends a more favorable prognosis than usual-type PCa. It has been proposed that the loss of p63 is associated with higher Gleason scores, an increased likelihood of metastasis, and worse prognosis in mouse metastasis models and in human clinical samples.[20] This is in concurrence with studies by Osunkoya et al., Baydar et al., and Giannico et al., which showed organ-confinement of p63-pCa in 100% (8 of 8), 100% (1 of 1), and 76% (16 of 21) of radical prostatectomy cases, respectively.[3],[4],[7] There were no lymph node metastases in all 12 of 21 cases with lymph node dissection.[7]
Although majority of cases had an overall Gleason score of ≥8 in the study of Giannico et al.,[7] mean Ki-67 expression was low (<5%) in all p63-PCa cases with similar expression in the coexisting acinar-type carcinoma. Low Ki-67 (6.25%) was also observed in another study.[3] Due to the discordance between the Gleason score and biologic behavior of p63-pCa, the use of the Gleason grading system may potentially lead to overtreatment. This raises the question on whether these tumors should be assigned a Gleason score.
It is important to make a distinction between aberrant nuclear and cytoplasmic expression of p63. A prospective study among 298 men, who were diagnosed with prostate cancer with predominantly cytoplasmic staining for p63-positive tumor cells, revealed an increase in prostate cancer-specific mortality with increasing expression of cytoplasmic p63 (tertiles). The shift in p63 localization may alter p63 stability, leading to disrupted cell cycle arrest and apoptosis.[21] In regard to the role of p63 in prostate development and tumorigenesis, another protein, aldehyde dehydrogenase 1A (ALDH1), has been reported to be associated with aberrant cytoplasmic p63 expression. In this study 18 out of 45 prostate cancer patients have high expression of cytoplasmic p63. They also reported that higher level of cytoplasmic p63 expression is correlated with higher proliferation by using Ki-67 staining.[22] This is in contrast to the low Ki-67 expression observed in aberrant nuclear p63 expression.
Currently, the prognostic significance of p63-pCa is still not well established. Additional studies are warranted to fully understand the biologic behavior of p63-pCa.
In conclusion, despite representing a rare variant of prostatic adenocarcinoma, recognition of aberrant diffuse p63 expression is critical because its confusing immunohistochemical staining pattern may be misinterpreted as simply benign or atypical especially when the lesion is focal and minute; and when p63/HMWCK basal cell cocktails are used. Pathologists should maintain a high index of suspicion for malignancy when infiltrative architecture and nuclear atypia are observed. In contrast to classic type prostatic adenocarcinomas, p63-PCa exhibit mixed luminal/basal immunophenotype; uniformly lack ERG gene rearrangement, SPINK1 expression, and PTEN loss; and frequently express GSTP1. Despite having unfavorable Gleason patterns, most of these tumors are found to be organ-confined on radical prostatectomy. With the discordance between their Gleason scores and biologic behavior, Gleason grading of these tumors remains debatable.
All efforts to secure patient's consent have been exhausted. The patient's anonymity is ensured. No other identifiers were included.
All authors certified fulfillment of ICMJE authorship criteria.
The authors declared no conflict of interest.
[1] Grisanzio C, Signoretti S. p63 in prostate biology and pathology. J Cell Biochem. 2008;103(5):1354–68. PubMed CrossRef.
[2] Humphrey PA. Diagnosis of adenocarcinoma in prostate needle biopsy tissue. J Clin Pathol. 2007;60(1):35-42. PubMed PubMed Central Crossref.
[3] Osunkoya AO, Hansel DE, Sun X, Netto GJ, Epstein JI. Aberrant diffuse expression of p63 in adenocarcinoma of the prostate on needle biopsy and radical prostatectomy: report of 21 cases. Am J Surg Pathol. 2008;32(3): 461-7. PubMed CrossRef.
[4] Baydar DE, Kulac I, Gurel B, De Marzo A. A case of prostatic adenocarcinoma with aberrant p63 expression: presentation with detailed immunohistochemical study and FISH analysis. Int J Surg Pathol. 2011;19(1):131-6. PubMed CrossRef.
[5] Wu A, Kunju LP. Prostate cancer with aberrant diffuse p63 expression. Arch Pathol Lab Med. 2013;137:1179-84. PubMed CrossRef.
[6] Leung A, Ross F, Roberts M, Watson R, Smith D, Srinivisan B. Aberrant p63 expression in prostate adenocarcinoma: a potential diagnostic pitfall. Pathology. 2017;49(1):S77. CrossRef.
[7] Giannico GA, Ross HM, Lotan T, Epstein JI. Aberrant expression of p63 in adenocarcinoma of the prostate. Am J Surg Pathol. 2013;37 (9):1401-6. PubMed CrossRef.
[8] Epstein JI, Egevad L, Humphrey PA, Montironi R; Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the prostate: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38(8):e6-e19. PubMed CrossRef.
[9] Signoretti S, Pires MM, Lindauer M, et al. p63 regulates commitment to the prostate cell lineage. Proc Natl Acad Sci U S A. 2005;102(32):11355-60. PubMed PubMed Central CrossRef.
[10] Signoretti S, Waltregny D, Dilks J, et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157(6):1769-75. PubMed PubMed Central CrossRef.
[11] Su X, Chakravati D, Flores ER. p63 steps into the limelight: crucial roles in the suppression of tumorigenesis and metastasis. Nat Rev Cancer. 2013;13(2):136-43. PubMed PubMed Central CrossRef.
[12] Parsons JK, Saria EA, Nakayama M, et al. Comprehensive mutational analysis and mRNA isoform quantification of TP63 in normal and neoplastic human prostate cells. Prostate. 2009;69(5):559-69. PubMed PubMed Central CrossRef.
[13] Tan H, Haffner MC, Esopi DM, et al. Prostate adenocarcinoma aberrantly expressing p63 are molecularly distinct from usual-type prostatic adenocarcinomas. Mod Pathol. 2015;28(3):446-56. PubMed PubMed Central CrossRef.
[14] Uchida K, Ross HM, Lotan T, et al. ΔNp63 (p40) expression in prostatic adenocarcinoma with diffuse p63 positivity. Hum Pathol. 2015 Mar; 46(3):384-9. PubMed CrossRef.
[15] Takahashi H, Fukutome K, Watanabe M, et al. Mutation analysis of the p51 gene and correlation between p53, p73, and p51 expressions in prostatic carcinoma. Prostate. 2001;47(2):85–90. PubMed CrossRef.
[16] Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–8. PubMed CrossRef.
[17] Tomlins SA, Rhodes DR, Yu J et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell. 2008;13(6):519–28. PubMed PubMed Central CrossRef.
[18] Krohn A, Diedler T, Burkhardt L, et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am J Pathol. 2012;181(2):401–12. PubMed CrossRef.
[19] Lee WH, Isaacs WB, Bova GS, Nelson WG. CG island methylation changes near the GSTP1 gene in prostatic carcinoma cells detected using the polymerase chain reaction: a new prostate cancer biomarker. Cancer Epidemiol Biomark Prev. 1997;6(6):443-50. PubMed.
[20] Tucci P, Agostini M, Grespi F, et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A. 2012; 109(38):15312-7. PubMed PubMed Central CrossRef.
[21] Dhillon PK, Barry M, Stampfer MJ, et al. Aberrant cytoplasmic expression of p63 and prostate cancer mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(2):595–600. PubMed Central NIHMSID: NIHMS107808.
[22] Ferronika P, Triningsih FE, Ghozali A, et al. p63 cytoplasmic aberrance is associated with high prostate cancer stem cell expression. Asian Pac J Cancer Prev. 2013;13(5):1943-8. PubMed.