Introduction. Mycobacterium tuberculosis and Mycobacterium leprae are acid-fast organisms with lipid-rich cell walls that resist decolorization with acidified alcohol after application of a dye with heat. The Ziehl-Neelsen and Fite Faraco staining technique, which are diagnostic tools for identification of acid-fast bacilli (AFB) found in histopathologic samples, are based on this principle. A modification of the Ziehl-Neelsen technique is described as an alternative rapid and reliable method of diagnosis for prompt detection and treatment.
Methodology. One hundred and seven (107) archived tissue specimens from autopsy and dermatology cases interpreted as positive for M. tuberculosis and M. leprae were stained using the proposed modified acid-fast (MAF) technique compared with Fite Faraco (FF) staining method as reference standard. Each specimen was read by two independent evaluators.
Results. The degree of diagnostic agreement of the MAF with FF was calculated. For autopsy (n=16) and dermatology (n=91) samples, the Cohen’s kappas are 0.765 (substantial) and 0.397 (fair), respectively. Overall, the Cohen’s kappa is 0.458 (moderate).
Conclusion. The proposed modified Acid-Fast staining method may be considered as an alternative to the conventional Ziehl-Neelsen method and the Fite Faraco method in identifying positive acid-fast bacilli in tissue samples taken from clinical cases of M. tuberculosis and M. leprae.
Key words: acid-fast bacilli, Mycobacterium tuberculosis, Mycobacterium leprae, tuberculosis, leprosy, Ziehl-Neelsen, Fite Faraco
Tuberculosis and leprosy remain among the world’s top infectious diseases.[1],[2],[3] It has been estimated that about one–third of the world’s population is infected with tuberculosis. It has killed nearly two million people each year and is the second leading cause of death worldwide among communicable diseases.[4] In the Philippines, it is the sixth leading cause of death and illness. In 2011, the World Health Organization (WHO) estimated 260,000 incident cases in the country, and that 28,000 people inflicted with the disease, die in a year.[5]
Leprosy on the other hand, has a global registered prevalence of 176,176 cases at the end of the year 2015. In the same year, the number of new cases reported was 211,973. The number of new cases indicates the intensity of the continued transmission of the disease.[6] In 2010, the Philippines had 2,041 new cases detected and 2,873 prevalent cases, while the Western Pacific Region registered 5,055 and 8,386 cases, respectively.[7]
Laboratory diagnosis of Mycobacterium leprae is generally made by microscopic and histopathological examination of slit skin smears. The Fite Faraco technique is the oldest method used to detect Mycobacterium leprae in tissue specimens. This technique, however, has been shown to have low sensitivity ranging from 40% to 70%,[8] is more complex, and takes two and a half hours of staining time from the principal investigator’s clinical experience.
Mycobacterium tuberculosis is diagnosed using the Ziehl-Neelsen Stain/Acid-Fast Stain which differentiates acid-fast from non-acid-fast bacilli. Although microbiological culture remains as the gold standard for this type of Mycobacterial infection, it takes 1 hour and 15 minutes, and has a limited sensitivity and specificity.[9] Histopathology remains an important method in diagnosis of the disease.
This study aims to determine the diagnostic agreement of a Modified Acid-fast (MAF) staining technique in the histopathological diagnosis of acid-fast bacilli (AFB) in tissue specimens, compared with the Fite Faraco (FF) staining method.
The study was conducted at the Research Institute for Tropical Medicine (RITM). One hundred and seven (107) formalin-fixed paraffin-embedded archived tissue specimens from previous autopsy and dermatology cases from 2003–2016 were used. Out of the 107 specimens, 16 were from excision during autopsy from different organs of patients who expired from M. tuberculosis, and 91 were obtained by punch biopsy from skin lesions of patients seen at the Institute’s dermatology clinic with impression of M. leprae.
The tissue samples were processed by standard paraffin wax techniques. Each tissue specimen was cut into three sections, 3-4 micra (µ) in thickness. A 3-µ tissue thickness was utilized for big tissue specimens, such as autopsy and biopsy specimens, while a 4-µ tissue thickness was utilized for small tissue specimens. One tissue section was utilized for the Fite Faraco Stain, and another section for the Modified Acid-fast Stain. The third section was intended to be used as a back-up sample, in the event that the tissue is sloughed off and the process is to be repeated.
Fite Faraco Stain
The requirements for the FF stain were the following solutions: (1) xylene–peanut oil solution, which is 1 part peanut oil (local brand) and 2 parts xylene (Merck), (2) carbol fuchsin solution: 2.5 ml melted phenol crystal (BDH), 5.0 ml absolute alcohol (Univar), 0.5 gm basic fuchsin (Merck), and 50 ml distilled water, (3) 1% hydrochloric acid solution: 100 ml 70% alcohol (Univar) and 1 ml concentrated hydrochloric acid (Merck), (4) methylene blue solution (Stock): 1.4 g methylene blue (Merck) and 100 ml 95% alcohol (Univar), (5) methylene blue solution (working): 10 ml methylene blue stock Solution and 90 ml distilled water.
The tissue sections were first deparaffinized through two changes of xylene–peanut oil solution for 12 minutes each and hydrated with distilled water. After draining, the excess oil was blotted to capacity. Thereafter, it was stained in carbolfuchsin stain for 1 hour at 25-35OC room temperature, and washed in tap water for 3 minutes. The slides were then differentiated individually with 1% hydrochloric acid for one minute until sections were faint pink by visual inspection. It was again washed in tap water for 3 minutes, before counterstaining with working methylene blue solution until light blue color is achieved. Excess methylene blue was rinsed off using tap water. Slides were then dipped in xylene before mounting with a resinous mounting medium. The specimen slides were screened for acid-fast bacilli under the microscope. The acid-fast bacilli stain bright red, while nuclei and the background stain blue.
Modified Acid-fast Stain
The solutions needed for the modified Ziehl-Neelsen stain (Z-N) were the following: (1) carbol fuchsin solution: 2.5 ml melted phenol crystals (BDH), 5.0 ml 100% alcohol (Univar), 0.5g basic fuchsin (Merck), 50 ml distilled water, (2) 0.3% acid alcohol (Univar), (3) methylene blue Solution (Stock): 1.4g methylene blue (Merck), 100 ml 95%alcohol (Univar), (4) Methylene Blue Solution (Working): 10 ml methylene blue Stock Solution, 90 ml distilled water.
The tissue sections were first deparaffinized, each dipped 3-4 times in descending grade of ethanol, and hydrated with distilled water. The tissue section was immersed in carbol fuchsin solution in a Coplin staining jar and heated for 30 minutes at 63 degrees Celsius in a constant temperature oven. The stain was washed off with running water before decolorizing with 0.3% acid alcohol, 2-4 dips until a faint pink color was achieved, depending on the thickness of the tissue. It was again washed in running water, with excess stain drained off, before counterstaining with methylene blue. Counterstaining was done in 5-6 dips, depending on the stain uptake of the tissue. The tissue section was washed again with tap water and rinsed in distilled water to remove mineral deposits, contaminants and other impurities. It was then dehydrated in 95% ethanol for 2-3 dips. Slides were then dipped in two changes of xylene for 5 minutes each before mounting with a resinous mounting medium. The acid-fast bacilli stain red to bright red, while non-acid-fast organisms are expected to stain blue.
Slide Interpretation
The samples from each specimen were randomly numbered so that the examiners were blinded to the sample identities, thereby ruling out bias. Before the start of the sample reading, inter-rater reliability was measured for the three independent evaluators using 20 pairs of randomly selected slides, separate from the actual slides in the study. The actual inter-rater reliability was 75.7%, using the intra-class coefficient reliability function of SPSS.
Each of the tissue specimen was read by two independent evaluators under oil immersion microscopy. Bacterial load was determined through quantitative microscopy of the slides under 100X oil immersion lens. The entire area of the section was examined at 100X magnification carefully. The sample is considered positive when one or more acid-fast bacteria is detected in at least one area of the tissue sample. If the bacilli were seen as purple or violet, the staining procedure was repeated with a back-up slide. If there is a difference in the reading of the two evaluators, a third evaluator read the specimen.
Data Analysis
The degree of agreement of the MAF staining method, compared with FF Staining method as the reference standard, was evaluated using Cohen’s kappa. This statistical method is useful for analyzing the agreement between two methods applied to the same sample, especially if one is considered the reference standard and the other is an alternative method.[10],[11]
Ethical Considerations
The study was reviewed by the RITM Institutional Research Board. The samples used in the study were de-identified, so as to protect the anonymity of patients. Only the researchers were able to access the samples in the laboratory.
Table 1 shows the crosstabulation of the results of the readings using the MAF compared to the FF method as the reference standard, including a separate cross tabulation for autopsy and dermatology samples. Table 2 summarizes the measures and interpretation of diagnostic agreement11 for the entire sample, and separately for the two different sample groups.
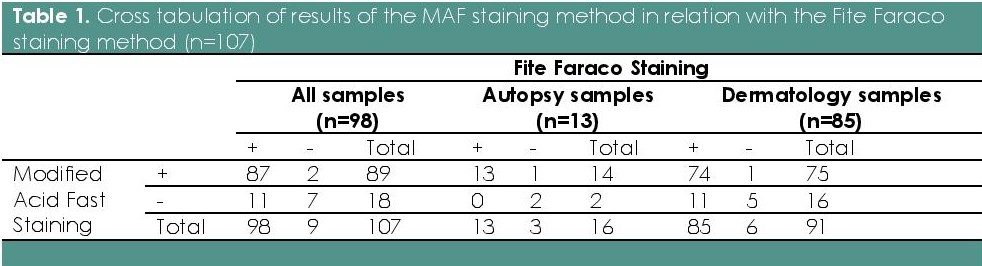
Table 1. Cross tabulation of results of the MAF staining method in relation with the Fite Faraco staining method (n=107)
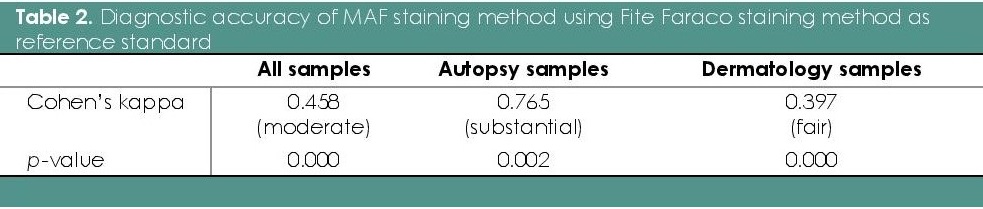
Table 2. Diagnostic accuracy of MAF staining method using Fite Faraco staining method as reference standard
Results showed that the MAF staining method showed moderate overall diagnostic accuracy, compared with the Fite Faraco method. The autopsy and dermatology samples were separated to examine the high number of false negative results in the over-all analysis. All the 11 false negative results were from the dermatology samples. As a result, the MAF staining method for autopsy samples had substantial diagnostic accuracy, compared with dermatology samples.
Figures 1 to 4 illustrate the staining results for the dermatology and autopsy samples using the Modified Acid-fast stain and the Fite Faraco stain.

Figure 1. Dermatology sample stained using the Modified Acid-fast procedure under oil immersion (100X).
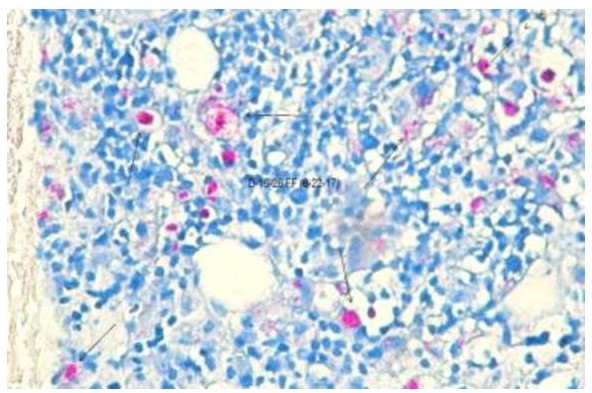
Figure 2. Dermatology sample stained using the Fite Faraco procedure under oil immersion (100X).
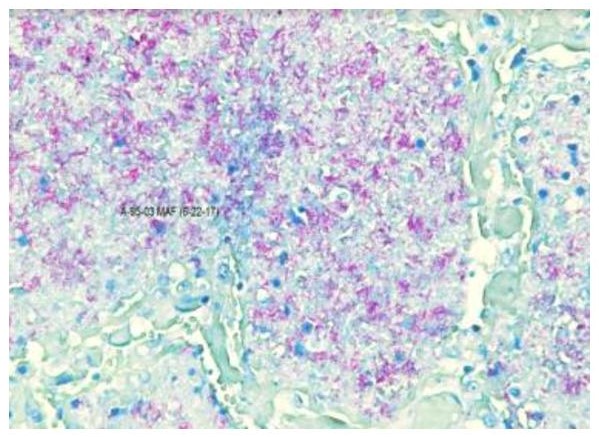
Figure 3. Autopsy sample stained using the Modified Acid-fast procedure under oil immersion (100X).
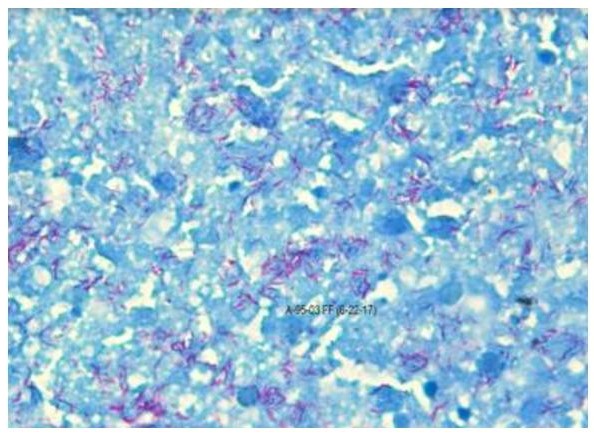
Figure 4. Autopsy sample stained using the Fite Faraco procedure under oil immersion (100X).
After establishing the diagnostic accuracy of the MAF staining method in randomly selected histopathology samples, the two staining methods were performed on 10 autopsy negative controls and 15 dermatology negative controls to identify false positive results that can be attributed to inappropriate reaction of the staining procedure. These negative controls were collected from samples that did not have a M. tuberculosis or M. leprae clinical diagnosis. None of these negative samples were found to be positive using the MAF and Fite Faraco staining methods.
The MAF staining method is a modification of the Ziehl-Neelsen (Z-N) method which the primary investigator developed during her experience in the Histopathology Laboratory of RITM. This modified method involved samples that are processed for 30 minutes at 63° Celsius in a constant temperature oven, instead of 1 hour under ambient temperature. Instead of using 2% Sulfuric Acid as the decolorizing agent for one minute, 0.3% Hydrochloric Acid was used for 5 seconds. Lastly, counterstain and dehydration times were done for only five seconds, which are shorter by 40 seconds compared to the conventional method.
Some samples positive for FF were negative in the MAF. This may be due either to the thickness of the cut or low bacterial load (i.e., paucibacillary specimens). Initially, there were 11 cases cut at 3µ which were positive for FF but negative in the MAF. These tissues were recut at a thicker 6µ using the microtome. Thereafter, only 3 positive for FF were negative in the MAF, supporting the earlier hypothesis.
These findings can be related to the MAF being similar to the Z-N method, which is known to have high sensitivity and positive predictive value, but with lower specificity.[12],[13],[14] The main goal of the Z-N method is to differentiate an AFB from a non-AFB, though not all Mycobacterium species can be detected well with this method. M. tuberculosis and M. ulcerans are strongly acid-fast which Z-N technique can be best used. FF, on the other hand, is more appropriate for M. leprae, which is weakly acid-fast.[9],[15],[16],[17] The Z-N method also requires a high bacterial load (5000–10000 AFB/ml) for detection, which may result to false negative results. Deparaffinization with peanut oil and xylene mixture with the Fite Faraco method protects the waxy coat of the bacilli which prevents shrinkage and disappearance during the process.[16],[18] Finally, the acid-fast property of Mycobacteria can also be affected by the age of the colonies, exposure to ultraviolet light and the heating process involved, and the medium where the bacteria were cultured.[19]
The shortened dyeing time with the carbol fuchsin from 1 hour to 30 minutes showed positive results because, regardless of the time, once stained, these microorganisms are resistant to destaining and cannot be decolorized easily with acid-alcohol solutions. Moreover, the added heat in the procedure enhanced the penetration of the carbol fuchsin dye through the bacterial cell wall and into the cytoplasm.[9],[15],[20],[21]
Using hydrochloric acid instead of sulfuric acid as a decolorizing agent also showed to be effective. The Revised National Tuberculosis Programme recommends the use of sulfuric acid as the decolorizing agent as it easily removes background material even from thick smears making identification of AFB easier.[22],[23],[24] On the other hand, the World Health Organization recommends the use of hydrochloric acid in alcohol to provide clean smears and enhance smear positivity, instead of sulfuric acid which was reported to produce unclean smears that can lower smear positivity for AFB.[22],[24] Various studies comparing the two agents showed that smears using hydrochloric acid as decolorizing agent have higher sensitivity and specificity compared with those using sulfuric acid.[22],[23],[24] In other researches, hydrochloric acid is as good as sulfuric acid as a decolorizing agent.23,25 In addition, hydrochloric acid is more economical, less costly, easier and safer to dilute and use.[24],[25],[26]
A shorter period for counterstaining compared with the conventional Z-N method was also observed to be effective with the prevention of masking and turning the bacilli purple.[27]
Several limitations were faced in this study, such as the use of archived tissues from past cases. An appropriate gold standard testing (e.g. PCR) was not done with the samples, which precluded the conduct of tests for sensitivity and specificity of the modified acid-fast staining procedure. Future studies should utilize slide blocks cut at 6µ using the microtome to limit false negative results and compare the results of the modified acid-fast staining procedure with PCR of the specimen.
The Modified Acid-fast staining method showed potential as an alternative to the Fite Faraco method in detecting AFB in tissues.
The authors thank the following for their valuable collective input in this paper, Remigio M. Olveda, MD, FPCP, FPSG, and Edelwisa S. Mercado, PhD, for their technical guidance in writing this paper; Laboratory Research Division Office, RITM for providing financial support in the development of the research protocol and coordinating the administrative concerns for this study; Catherine Masangkay, MD DPSP for supporting the primary author to conduct the research study and data collection procedure in the laboratory; Teresita Gabriel, MD, Head, of the Dermatology Department; for allowing access to archived tissue blocks. Evariza L. Jorda, RMT and Rachel E. Estrabo, RMT for providing laboratory support during data collection; Ireneo T. Balighot for providing assistance in the disposal of used reagents and cleaning the equipment after the data collection procedure; Dan Louie Renz P. Tating, RN, for providing expert direction and advice throughout the conduct of the study until the writing of the manuscript; and Ericka Louise C. Gilo, RN and Hannah Kristine Mugol, RN for assisting in providing support for the writing of the proposal and manuscript.
All authors certified fulfillment of ICMJE authorship criteria.
The authors declared no conflict of interest.
None.
[1] Rose AH. Chemical microbiology: an introduction to microbial physiology. USA: Elsevier, 1976.
[2] World Health Organization. Tuberculosis: WHO Fact Sheet 104. Accessed November 25, 2016.
[3] World Health Organization. Leprosy: WHO Fact Sheet 101. Accessed November 25, 2016.
[4] Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003; 13;362(9387):887-99. PubMed CrossRef
[5] WHO-Western Pacific Region. Tuberculosis. http://www.wpro.who.int/philippines/areas/communicable_diseases/tb/story_continuation_tb_area_page/en/. Accessed November 25, 2016.
[6] World Health Organization. Epidemiology: leprosy. Accessed November 25, 2016.
[7] WHO-Western Pacific Region. Leprosy control and the burden of leprosy in the Philippines: 2006-2010. Available at: http://www.wpro.who.int/philippines/areas/communicable_diseases/leprosy/who_leprosy_control_burden_.pdf..
[8] Schlossberg D, ed. Clinical infectious disease. New York: Cambridge University Press, 2008.
[9] Acharya T. Ziehl-Neelsen technique (AFB staining): principle, procedure and reporting. Bacteriology, laboratory diagnosis of bacterial disease staining techniques in microbiology, 2013. Retrieved from Microbe Online: http://microbeonline.com/ziehl-neelsen-technique-principle-procedure-reporting/.
[10] Watson PF, Petrie A. Method agreement analysis: a review of correct methodology. Theriogenology. 2010;73(9):1167-79. CrossRef
[11] Landis JR., Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;3(1):159-74. PubMed
[12] Balakrishna J, Shahapur P, Chakradhar P, Saheb H. Comparative study of different staining techniques -Ziehlneelsen stain, Gabbet’s stain, Fluorochrom stain- for detecting of mycobacterium tuberculosis in the sputum. J Pharm Sci Res. 2013;5(4):89-92.
[13] Karadağ A, Usta E, Bilgin K, etc. Comparison of culture, real-time DNA amplification assay and Ehrlich-Ziehl-Neelsen for detection of mycobacterium tuberculosis. Balkan Med J. 2013;30:13-5. CrossRef
[14] Murray SJ, Barrett A, Magee JG, Freeman R. Optimisation of acid-fast smears for the direct detection of mycobacteria in clinical samples. J Clin Pathol. 2003;56(7):613-5. PubMed PubMed Central
[15] Tille P. Mycobacteria and other bacteria with unsual growth requirements. In: Bailey and Scott's Diagnositc Microbiology, 13th ed. St. Louis, Missiouri: Mosby, Inc., 2014.
[16] Reija AH, Biswas N, Biswas S, et al. Fite-faraco staining in combination with multiplex polymerase chain reaction: A new approach to leprosy diagnosis. Indian J Dermatol Venereol Leprol. 2013;79(5):693-700. PubMed CrossRef
[17] Sandhika AW, Adriaty D, Agusni I. Detection of mycobacterium leprae in formalin-fixed paraffin-embedded sample by fite-faraco staining and polymerase chain reaction. Elsevier Procedia Chemistry. 2016;18:231-6. CrossRef
[18] Lydard P, Cole M, Holton J, et al. Case studies in infectious disease: Mycobacterium leprae. New York: Garland Science, Taylor and Francis Group, LLC, 2009.
[19] Murray SJ, Barrett A, Magee JG, Freeman R. Optimisation of acid-fast smears for the direct detection of mycobacteria in clinical samples. J CLin Pathol. 2003;56(8):613-5. PubMed CrossRef
[20] Dalynn Biologicals. Carbol fuchsin stain (Ziehl-Neelsen), 2004. http://www.dalynn.com/dyn/ck_assets/files/tech/SC25.pdf. Accessed November 25, 2016.
[21] Zhao D, Yang XM, Chen QY, Zhang XS, Guo CJ, Che XY. A modified acid-fast staining method for rapid detection of mycobacterium tuberculosis. J Microbiol Methods. 2012;91(1):128-32. PubMed CrossRef
[22] Chandra TJ, Raj RS, Sharma YV. Hydrochloric acid vs. sulfuric acid - an economical destaining reagent for Ziehl Neelsen staining to detect acid-fast bacilli in sputum smears. Int J.Bioassays. 2014;3(8):3241-3.
[23] Sekar MG, Rehman F, Kumar V, Selvakumar N. Equivalence of acid alone or acid-alcohol as decolourizing agent in Ziehl-Neelsen method. Indian J Tuberc. 2012;59(4):219-23. PubMed
[24] Chandra T, Raj R, Sharma YV. Same day sputum smear microscopy approach with modified ZN staining for the diagnosis of pulmonary tuberculosis in a microscopy centre at Rajahmundry. Indian J Med Microbiol 2014;32(2):153-6. PubMed CrossRef
[25] Mokhtari Z, Larbaoui D. Corrélation entre les résultats d‘unexamen direct très faiblement positif et ceux de la culture—résultats préliminaires. Bull Union Int Tuberc. 1973;48:88-89.
[26] Aung KJ, Nandi P, Hamid Salim A, Hossain A, Van Deun A. Hydrochloric vs. sulphuric acid in water for Ziehl-Neelsen staining of acid-fast bacilli. Int J Tuberc Lung Dis. 2011; 15(7):955-8. PubMed CrossRef
[27] Nayak SV, Shivarudrappa AS, Nagarajappa AH, Sacchidanand S, Ahmed SM. Role of modified rapid AFB method in histopathological sections of Hansen's disease. Indian J Dermatol Venereol Leprol. 2003;69(2):173-4. PubMed