Background. Epidermal Growth Factor Receptor (EGFR) mutation status has been shown to have a significant prognostic and predictive role in the management of Non-small Cell Lung Carcinoma (NSCLC), significantly prolonging patients' survival. Thus, EGFR mutational analysis before initiation of treatment is now recommended in several clinical practice guidelines. Although EGFR mutation testing in NSCLC has been a part of clinical care in the Philippines, there is little data on the EGFR mutation spectrum among Filipinos.
Objective. This study aims to determine the frequency of EGFR mutations among Filipino population diagnosed with NSCLC in a private tertiary care setting.
Methodology. A total of 626 tissue samples (444 biopsies, 108 pleural/ascitic fluids, 74 excision/resection), during a 15-month period (January 2015-March 2016) were assessed for the known EGFR driver mutations (exons 18, 19, 20, 21) using the Roche EGFR protocol with the Cobas Quantitative Real Time PCR. Macrodissection was performed as necessary. Available patient demographics were recorded. Statistical analyses were performed using the Fisher's exact test.
Results. In this study, we report the largest EGFR mutation profiling data among Filipino patients with NSCLC, which showed an overall 49.4 % EGFR mutation rate. The mutation rates according to histologic types, were as follows: adenocarcinoma (49.9 %, n=287/575), squamous cell carcinoma (3.5%, n=9/26), NSCLC NOS (50 %, n=10/20), adenosquamous cell carcinoma (66.7 %, n=2/3), and adenocarcinoma with neuroendocrine features (50%, n=1/2). Consistent with the literature, we found a significant higher incidence of EGFR mutation among women than men (60.2% vs 39.8%). With regards to individual mutation types, the most common mutations detected were deletions in exon 19 (54.7 %, n=168), followed by L858R point mutation in exon 21 (27.4 %, n=84).
Conclusion. The incidence of EGFR mutations in NSCLC varies across different ethnicity. In previous reports, the frequency of EGFR mutations is approximately 30% (with a range of 22.2% to 64.2%) among the Asian population compared with 20% among the white population. In the Philippines, the incidence of EGFR mutations is sparsely explored. Here we report the largest EGFR mutation profiling data among Filipinos with NSCLC in a tertiary care setting, with a frequency of 49.4%. This prevalence is almost similar to those reported in Asia. EGFR is differentially mutated among NSCLC patients with different gender, as women have significantly higher incidence than men. Hence, this study establishes relevance of routine EGFR mutation testing for all NSCLC patients as part of initial workup at diagnosis and underscores the significant role of EGFR inhibitors as a treatment option among Filipino population.
Key words: Epidermal growth factor receptor, non-small cell lung carcinoma, Exons 18,19,20 and 21, T790M, polymerase chain reaction
Lung cancer is the leading cause of cancer mortality in the world and is the second most common cause of cancer deaths among Filipinos. Approximately 85% of lung cancer cases are non-small lung cancers (NSCLC).[1] In patients with NSCLC, platinum-based chemotherapy used to be considered as standard first-line treatment.[2] During the recent years, there has been continuous development of new and effective targeted treatment modalities for advanced NSCLC. One of these therapeutic agents are tyrosine kinase inhibitors (TKI) which targets mutant epidermal growth factor receptor (EGFR).[3]-[4] EGFR mutations play an important role in the pathogenesis of multiple carcinoma, including NSCLC. Activating EGFR mutation promotes tumor growth and progression, stimulates tumor cell proliferation, inhibits apoptosis and produces angiogenic factors.[5] For patients with advanced NSCLC harboring EGFR mutation, several phase III studies have shown the clinical efficacy of the FDA approved EGFR inhibitors, gefitinib, afatinib and erlotinib, as compared to platinum-based chemotherapy when used as first line of treatment. Also, EGFR mutation status has been shown to have a significant prognostic and predictive role in the management of NSCLC, significantly prolonging patients' survival.[6],[7],[8] Not all types of EGFR mutation, however, are responsive to the first-generation tyrosine kinase inhibitors. For NSCLC patients with the common EGFR mutations, namely exon 19 deletion and exon 21 L858R, the response rate to TKIs (gefitinib and erlotinib) is approximately 60%. Several studies also suggested that patients with exon 19 deletion mutation might be more sensitive to targeted therapy than with exon 21 L858R. On the contrary, mutation in exon 20 (T790M) have been associated with resistance to first generation TKIs. EGFR T790 mutations usually occurs as a resistance mutation after first generation TKI therapy. For patients with EGFR T790M mutations, treatment with osimertinib may be effective and nazartinib (EGF816) is promising for the majority of them.[9] Thus, an accurate EGFR mutational analysis before initiation of treatment and repeat EGFR mutation testing at relapse, are now recommended in several clinical practice guidelines.[11]
The incidence of EGFR mutations in NSCLC varies across different ethnicities. In previous reports, the frequency of EGFR mutations is approximately 30% (range of 22.2 % to 62%) among the Asian population compared with 20% among the white populations.[11],[12] In the Philippines, although EGFR mutation testing in NSCLC has been a part of clinical care setting, there is little data on the EGFR mutation spectrum among Filipinos. Here, in this study we determine the EGFR mutation status in 626 NSCLC patients of Filipino ethnicity and correlate with different variables like age, gender, and histologic types.
This is a retrospective study of Filipino patients diagnosed with NSCLC who were referred to our institution for EGFR mutation testing from various hospitals across the Philippines over a 15-month period (January 2015-March 2016). The EGFR mutational status is performed when a diagnosis of adenocarcinoma, non-small cell carcinoma favors squamous cell carcinoma, adenosquamous carcinoma and non-small cell carcinoma, NOS are rendered. The histopathological diagnoses were mostly made based on the histomorphology with or without immunohistochemical staining performed with TTF-1, p63, Napsin A, CK7, CK20. In distinguishing adenocarcinoma from squamous cell carcinoma, or a lung primary vs. metastatic, 2 or more immunohistochemical stains were used. 78 of 626 patients were diagnosed as primary lung carcinoma using panel of immunohistochemical stains (positive for CK7, TTF1 and Napsin A; negative for CK20). 44 cases were diagnosed using positivity with TTF-1 only. The remaining cases were diagnosed as primary lung carcinoma based on clinical and radiologic correlation (absence of other organ involvement). The patient demographic data (including age, nationality, gender) if available, and histologic diagnosis were recorded. This study was approved by the Institutional Scientific Review Board and Ethics Committee of St. Luke's Medical Center-Quezon City. Since this was a retrospective analysis, the ISRB and the IERC waived the need for an informed consent.
Inclusion criteria
All Filipino patients diagnosed as Non-small cell Lung Carcinoma from St. Luke’s Medical Center, Global City and other hospitals from the Philippines.
Exclusion criteria
Formalin-fixed paraffin embedded (FFPE) samples from patients diagnosed with NSCLC containing less than 5 % viable tumor cells were excluded from this study. Patients with incomplete histopathologic report were also excluded from this study.
Collection of patient samples
The patient's FFPE blocks and H&E slides, together with histopathological report, were sent to the Cellular Immunology Section of the Institute of Pathology-St. Luke's Medical Center. The hematoxylin and eosin stained slides were viewed under the microscope to confirm that the tumor cells constitute more than 5% of the tissue mass. Macrodissection was performed on cases with less than 50 % tumor cells in the tissue block/slide. A total of 626 tissue samples (444 biopsies, 108 pleural/ascitic fluids, 74 excision/resection) were assessed for EGFR mutation.
DNA extraction
DNA was isolated from the FFPE samples after deparaffinization and extraction of 3-5mm thick paraffin sections in xylene. The Cobas Roche DNA Sample Preparation Kit (Roche Diagnostics, USA) and the Cobas cfDNA Sample Preparation Kit (Roche Diagnostics, USA) were used for manual sample preparations, which were based on nucleic acid binding to glass fibers.
Mutation Analysis by PCR and Sanger Sequencing
After the extraction of DNA, the target DNA was then amplified and detected on the Cobas Z 480 analyzer (Roche Diagnostics, USA) using the amplification and detection reagents provided in the Cobas Roche EGFR Mutation Test v1 kit (Roche Diagnostics, USA). A mutant control and negative control were included in each run to confirm the validity of the run.
The Cobas Roche EGFR Test is designed to detect the following mutations:
- Exon 18: G719X (G719A, G719C, and G719S) - Exon 19: deletions and complex mutations - Exon 20: S768I, T790M, and insertions - Exon 21: L858R and L861Q
Statistical analysis
The Fischer's exact test was performed to reveal any significant correlation between the mutation status and gender, gender and specific mutation type.
626 Filipino patients diagnosed with lung cancer were tested for common EGFR mutation subtypes by real time PCR using TaqMan primer probes for point mutations in exons 18, 20 and 21 and in frame deletion in exon 19. As shown in Table 1, of the 626 patients tested, 52% (n=325) were males and 48% (n=301) were females. The median age was 64 years (with a range of 13-94 years old). The overall EGFR mutational analysis result was positive in 49.4% (n=309), negative (wild type) in 50% (n=313) and invalid in 0.6 % (n=4). Invalid results were secondary to DNA degradation, resulting to failure of PCR amplification or testing. The presence of EGFR mutation was significantly higher in females (60.2%) as compared with males (39.8%) (two tailed p test, p<0.0001) (Table 2). As to histologic classification with EGFR mutation, there were 575 cases of adenocarcinoma, 26 cases of squamous cell carcinoma, 20 cases of NSCLC NOS, 3 cases of adenosquamous cell carcinoma and 2 cases of adenocarcinoma with neuroendocrine features. EGFR mutations were identified in 49.9% of adenocarcinoma (n=287), 3.5% of squamous cell carcinoma (n=9), 50% of NSCLC NOS (n=10), 66.7% of adenosquamous cell carcinoma (n=2), and 50% of adenocarcinoma with neuroendocrine features (n=1) (Table 1).
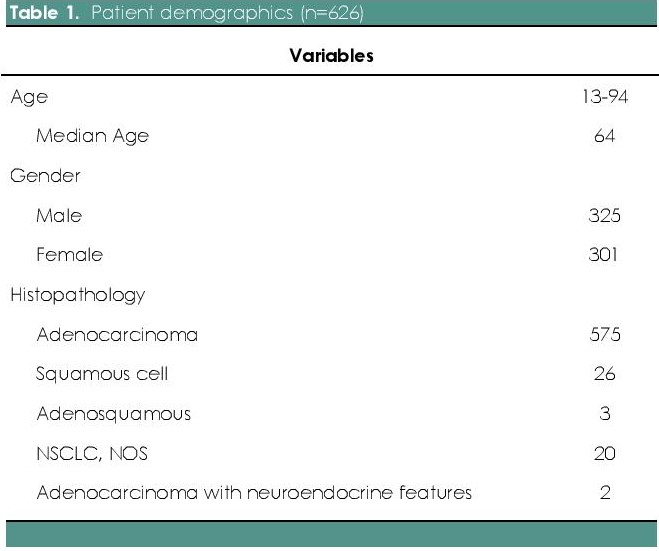
Table 1. Patient demographics (n=626)
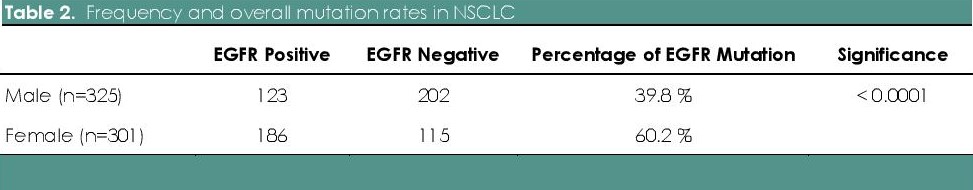
Table 2. Frequency and overall mutation rates in NSCLC
In our study, there were 26 patients less than 40 years old (male n=11, female n=15), 221 patients between 40-60 years old (male n=114, female n=107) and 379 patients older than 60 years old (male n=200, female n= 179). Although not statistically significant, the EGFR mutation rate was higher in patients with age >60 (Roche Diagnostics, USA) years as compared to < 60 years (61.4 % vs. 38.5 %) (p=0.0661). With respect to gender, mutation rate in females was higher in older individuals (>60 years) as compared to 40-60 years (66% vs. 32%; n=123 vs. 59), which was statistically significant (p=0.022789). In males, there was no statistical significance as to age group between 40-60 years and >60 years (54% vs. 41%; n=67 vs. 51; p value= 0.05313) (Tables 3a and 3b).

Table 3a. EGFR mutation status with respect to age and gender
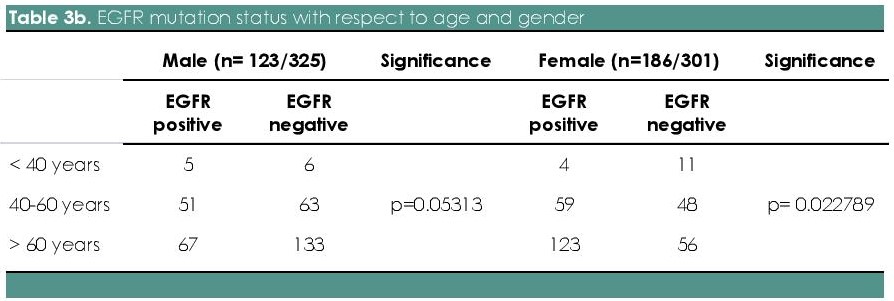
Table 3b. Table 3b. EGFR mutation status with respect to age and gender
Among the 309 EGFR mutated cases, 54.7 % (n=168) have deletions in exon 19, 27.4 % (n=84) with point mutations in exon 21 L858R, 7.2 % (n=22) with exon 20 insertion, 1.3% (n=4) with point mutation in exon 18 G719X, 0.7% (n=2) with point mutation in exon 18 L861Q and 0.3% (n=1) with point mutation in exon 20 T790M. Dual mutations in exons 19, 20 and 21 were found in 8.4 % (n=26) of the cases. Dual mutations involving exon 19 in combination with others was seen in 15 (4.9 %) patients; exon 21 L858R point mutations in combination with others was seen in 11 (3.6 %) patients. (Figure 1). Out of the 26 cases of squamous cell carcinoma, 9 cases (34.6 %) showed EGFR mutation, which include: exon 19 deletion (55.6 %, n=5/9), point mutations in exon 21 L858R (33.3 %, n=3/9) and exon 20 insertion (11.1 %, n=1/9). Exon 19 deletion is found to be the most common mutation among both males and females. But, the prevalence of mutations in exon 19 deletion (p=0.0069) and exon 21 L858R (p<0.0001) were significantly higher in females as compared with males (Table 4 and Figure 2). Between different age groups (<40 years, 40-60 years and >60 years), we did not find any statistical significance in terms of difference in the prevalence of EGFR mutation (p value-0.4815) (Figure 3).
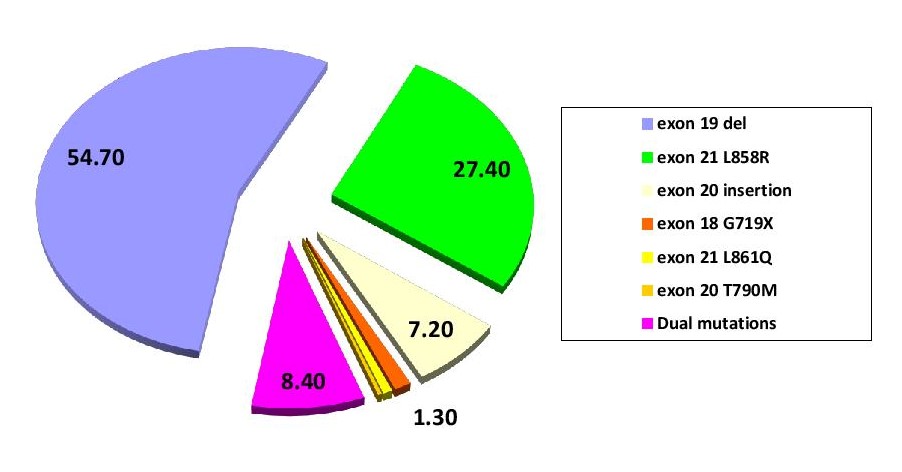
Figure 1. EGFR mutation status among NSCLC Filipino patients.
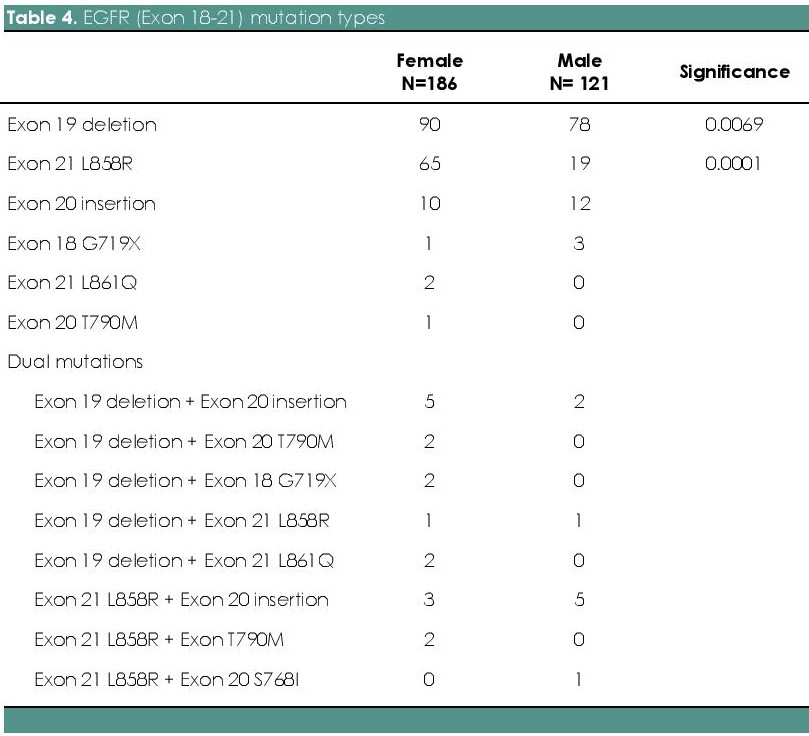
Table 4. EGFR (Exon 18-21) mutation types
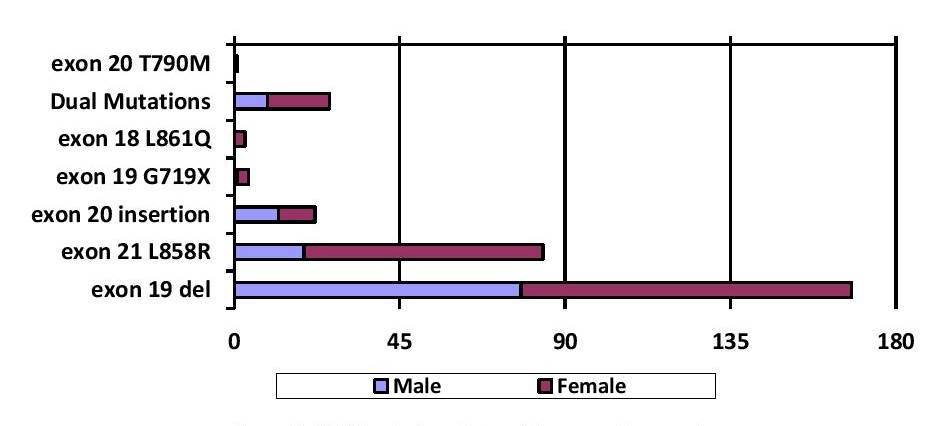
Figure 2. EGFR mutation status with respect to gender.
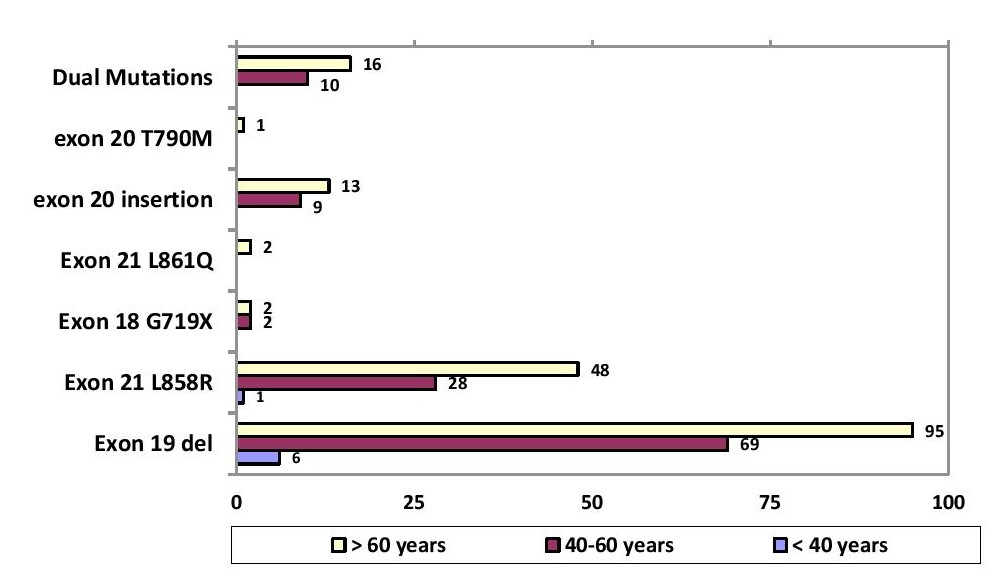
Figure 3. Specific EGFR mutation with respect to age group.
Because EGFR mutation status has been shown to have a significant prognostic and predictive role in the management of NSCLC, many countries have recommended an accurate EGFR mutational analysis before initiation of treatment to determine appropriate treatment with EGFR inhibitors. Several studies suggested that clinical response to TKIs with exon 19 del mutations might be more sensitive than with exon 21 L858R. In one study by Jiang-Yong Yu et al., of the 453 patients with mutations in Exon 19 del and Exon 21 L858R, the response rate of TKIs in patients with Exon 19 del was significantly higher than that with exon 21 L858R mutations (55.2% vs. 43.7%).[13] For rare genotypes, recent studies showed that these could be targetable if appropriate TKI are selected. For example, mutations with G719X, Del18, E709K, insertions in exon 19, S768I or L861Q have moderate sensitivities to gefitinib or erlotinib with response rate of 30%– 50%.[9] In up to 60%–80% of patients treated with TKIs, the tumor regresses dramatically, but after a median time of 9-12 months, all patients develop acquired resistance to the targeted therapy. A secondary mutation, T790M, in the exon 20 of the EGFR gene is the most frequent cause of acquired resistance, which is found in 50%–60% of relapsed cases. Among other causes of acquired resistance are: target-independent mechanisms such as MET amplification (4%), Human EGFR type 2 (HER2) amplification (8%–13%), PIK3CA mutation (2%), BRAF mutation (1%), histological transformation from NSCLC to SCLC (6%), or epithelial–mesenchymal transition (1%-2%). Unknown mechanism of acquired resistance is noted in 18% of cases.[14],[15],[16]
In the Philippines, the incidence of EGFR mutations is sparsely explored. In the PIONEER study, the authors have demonstrated that approximately half (51.4%) of the patients with NSCLC from seven regions of Asia harbored EGFR mutations. As with regards to Asian regions, Vietnam (64.2%) has the highest incidence while India has the lowest incidence (22.2%). Frequency of EGFR mutations was significantly higher among women (61.1%) than men (44%) (Table 5).[17],[18] In previous study from the Philippines, the EGFR mutation rate was also reported to be 42 %.[19]
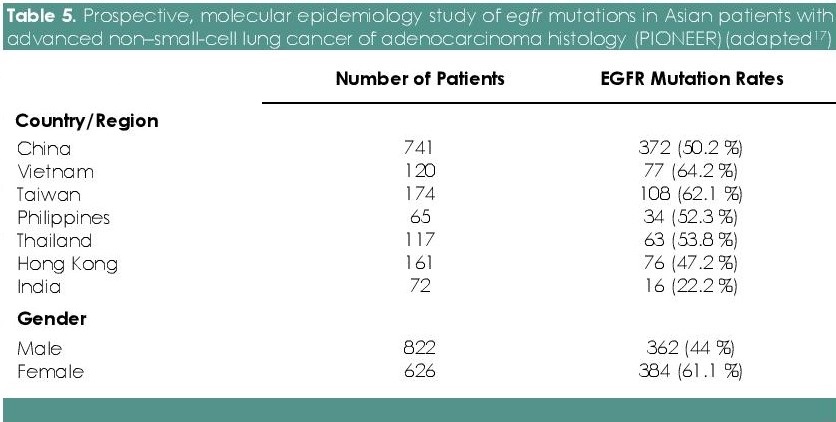
Table 5. Prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non–small-cell lung cancer of adenocarcinoma histology (PIONEER)(adapted[17])
In this study, we report the largest EGFR mutation profiling data among Filipino patients with NSCLC from a tertiary private hospital, which showed an overall 49.4 % EGFR mutation, mostly were adenocarcinoma. This prevalence is similar to those reported in Asia.[17] Consistent with the literature, we found a significantly higher incidence of EGFR mutation among women than men (62% vs 39.8%). Among patients with squamous cell lung carcinomas, there have been few studies within East Asians with conflicting results: two study from China reported EGFR mutation rate of 21% (3/14) and 13.3% (4/30) compared to a Korean based study with a mutation rate of 7.3% (3/41).[20],[21],[22] We found EGFR mutation rate of 35 % (9/26) in small cohort of 26 patients with squamous cell carcinoma. In which 4 of these cases were confirmed by immunohistochemical staining as histologically squamous cell carcinoma. Of these positive specimens, 6 came from FNAB/CT-guided biopsy of lung, 2 from bronchial biopsy and 1 lobectomy specimen. We cannot entirely rule out an adenocarcinoma component of Adenosquamous carcinoma in the cases where no immunohistochemical staining was performed. Nevertheless, rare occurrence of EGFR mutation among cases diagnosed as SQCC histologically warrants inclusion of these cases for EGFR mutation screening in routine practice.
With regards to individual mutation types, the most common mutation in these studies detected was deletion in exon 19 (54.7%) followed by L858R point mutation in exon 21 (27.4%), similar to that described in IPASS study. The IPASS study showed 53.6% had exon 19 deletions and 42.5% had a mutation at exon 21 (L858R).[23] The Pioneer study showed the following mutation rates: 24.6% for exon 19 deletion and 22.8% for L858R point mutation.
We found dual mutations in exons 19, 20 and 21 in 8.4% of the cases. In the Pioneer study, dual mutations were also seen: exon 19 deletion in combination with others comprising 24.3% [352 of 1450] and L858R point mutation in exon 21 alone in combination with others were 22.9% [332 of 1450].[17] But in the IPASS study, patients that were dually mutated were 4.2 % (11/261).[23] The variability of the incidence of dual mutations identified in these studies may be secondary to different diagnostic sensitivities of the different molecular platforms utilized.
Major limitations of this study are the following: correlation of EGFR mutation patterns with other clinical characteristics (e.g. smoking history, patient’s family history, grading and staging of NSCLC), complete information on targeted treatment received, response to targeted therapy and overall survival data in the Filipino population. Since the cases were collected from 2015-2016, survival data of these patients were far from maturity.
In summary, western population shows a mutation rate of 20%[11],[12] and Asian population show a heterogeneous mutation rate of 22–64%.[17]-[19] EGFR mutation rate of 49.4 % among Filipino population in our cohort is similar that of East Asian patients. EGFR mutations were significantly higher in females than males. Although EGFR mutation is common among younger patients, interestingly, we found mutation rate among females to be higher among older individuals (>60 years). This study warrants validation in a larger prospective study.
The incidence of EGFR mutations in NSCLC varies across different ethnicity. In previous reports, the frequency of EGFR mutations among the Asian population are approximately 30% (with a range of 22.2% to 64.2%) compared with the western population (20%). In the Philippines, the incidence of EGFR mutations is sparsely explored. Here we report the largest EGFR mutation profiling data among Filipinos with NSCLC in a private tertiary care setting, with a frequency of 49.4%. This prevalence is almost similar to those reported in Asia. EGFR is differentially mutated among NSCLC patients with different gender, as women have significantly higher incidence than men. With regards to individual mutation types, the most common mutations detected were deletion in exon 19 followed by L858R point mutation in Exon 21. Also, rare occurrence of EGFR mutation among cases diagnosed as squamous cell carcinoma histologically warrants inclusion of these cases for EGFR mutation screening.
Hence, this study establishes relevance of routine EGFR mutation diagnostics for NSCLC patients in the clinical setting and emphasizes effectiveness for adoption of EGFR inhibitors as a treatment among Filipino population. Further studies to correlate EGFR mutation patterns with other clinical characteristics (patient’s family history, smoking history, grading and staging of NSCLC), response to targeted therapy and overall survival in the Filipino population is warranted.
All authors certified fulfillment of ICMJE authorship criteria.
The authors declared no conflict of interest.
None.
[1] Ferlay JSH, Bray F, Forman D, Mathers C, and Parkin DM. GLOBOCAN 2012, Cancer incidence and mortality worldwide: IARC Cancer Base No. 10 (Internet). Lyon, France: International Agency for Research on Cancer.
[2] Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomized clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311(7010):899–909. PubMed PubMed Central
[3] Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. PubMed CrossRef
[4] Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. PubMed CrossRef
[5] Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor- related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995;9(3):183–232. PubMed
[6] Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–8. PubMed CrossRef
[7] Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362(25):2380–8. PubMed CrossRef
[8] Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–11. PubMed PubMed Central CrossRef
[9] Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci. 2016;107(9):1179–86.
[10] D’Addario G, Felip E,; ESMO Guidelines Working Group. Non-small-cell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(Suppl 2): ii39–40. PubMed CrossRef
[11] Kawaguchi T, Matsumura A, Fukai S, et al. Japanese ethnicity compared with Caucasian ethnicity and never-smoking status are independent favorable prognostic factors for overall survival in non-small cell lung cancer: a collaborative epidemiologic study of the National Hospital Organization Study Group for Lung Cancer (NHSGLC) in Japan and a Southern California Regional Cancer Registry databases. J Thorac Oncol. 2010;5(7):1001–10. PubMed CrossRef
[12] Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–67. PuBmed CrossRef
[13] Yu JY, Yu SF, Wang SH, et al. Clinical outcomes of EGFR-TKI treatment and genetic heterogeneity in lung adenocarcinoma patients with EGFR mutations on exons 19 and 21. Chin J Cancer. 2016; 35:30. PubMed PubMed Central CrossRef
[14] Romanidou O, Landi L, Cappuzzo F, Califano R. Overcoming resistance to first/second generation epidermal growth factor receptor tyrosine kinase inhibitors and ALK inhibitors in oncogene-addicted advanced non-small cell lung cancer. Ther Adv Med Oncol. 2016;8(3):176–87. PubMed PubMed Central CrossRef
[15] Cappuzzo F. Overcoming EGFR-TKI Resistance. Guide to Targeted Therapies: Treatment Resistance in Lung Cancer. Switzerland: Adis-Springer, 2015. CrossRef
[16] Jänne PA, Yang JC, Kim DW. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–99. PubMed CrossRef
[17] 17. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non–small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154 -62. PubMed PubMed Central CrossRef.
[18] Yang PC, Shi Y, Au JS, et al. Molecular epidemiological prospective study of EGFR mutations from Asian patients (pts) with advanced lung adenocarcinoma (PIONEER). J Clin Oncol. 2012;30. http://ascopubs.org/doi/abs/10.1200/jco.2012.30.15_suppl.1534..
[19] Heralde FI, Barzaga MT, Tan-Liu N, Bernal S. EGFR mutation profile of NSCLC patients tested at the Lung Center of the Philippines. J Thorac Oncol. 2017;12(11):S2257-8. Crossref
[20] Lee JW, Soung YH, Kim SY, et al. Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11(8):2879–82. PubMed CrossRef
[21] Li M, Zhang Q, Liu L, et al. The different clinical significance of EGFR mutations in exon 19 and 21 in non-small cell lung cancer patients of China. Neoplasma. 2011;58(1):74–81. PubMed
[22] Sun MH, Yang F, Shen L, et al. [Detection of epidermal growth factor receptor mutations in non-small-cell lung carcinoma by direct sequencing and correlations with clinicopathological characteristics and sample types]. Zhonghua Bing Li Xue Za Zhi, 2011;40(10):655–9. PubMed
[23] Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. PubMed Crossref