Contrast-enhanced Spectral Mammography (CESM) is an emerging and promising functional imaging modality that tries to address the paucity of physiologic-based tumor imaging for the detection of breast cancer.
This article describes two cases of women with non-dense and dense breasts presenting with clinically palpable breast masses and the depiction of breast cancer utilizing Contrast- enhanced Spectral Mammography.
Key words: Contrast-Enhanced Spectral Mammography (CESM), Digital Breast Tomosynthesis, Magnetic Resonance Imaging, Low energy, Subtracted Image, Full-Field Digital Mammography (FFDM)
Mammography is the only breast imaging modality with a demonstrated ability to reduce mortality.[1] A review by Myers et al., confirmed the findings of several published studies that screening mammography in women aged 40-79 reduces breast cancer mortality rates by 20%-50%, with extent of benefit varying by age, as well as study design (RCT vs. observational)[2] However, mammography has a population-based sensitivity of only approximately 80%1 which is further corroborated by the findings of Carney et al., that showed that with increasing breast density, the sensitivity of mammography decreases to 62% in women with dense breasts.[3]
Breast density refers to the proportion of glandular and fibrous breast tissue to the amount of fatty tissues in a woman’s breast. It has been shown that women who have high density breasts are 4-5x more likely to get breast cancer than women with low breast density.[4],[5] It is also statistically significantly greater among Asian women than among African American and white women.[6]
Contrast-Enhanced Spectral Mammography (CESM)
Contrast-enhanced Spectral Mammography (CESM) is a novel imaging modality that demonstrates the physiologic uptake of contrast by breast cancer. The depiction of breast cancers using contrast media is based on the biologic principle of the rapid formation of tumoral microvessels that render malignancy-associated vessels more permeable to contrast agent than normal tissue, resulting in tumor enhancement.[7]
It has been proposed by Chang et al., that the use of a standard iodinated CT contrast agent and x-ray imaging might also give functional information with a preferential uptake in breast cancers.[8] An early study done among 26 subjects in 2003 by Lewin et al., of the University of Colorado utilizing dual-energy contrast-enhanced mammography showed tumor enhancement in 13 of the subjects that had subsequent biopsy-proven invasive cancers.[9]
Contrast-enhanced Spectral Mammography was introduced in Europe in June 2010 and received FDA approval in the United States on October 2011. The first center in the UK to acquire the technology was Nottingham Breast Unit and in the United States, early adopters include Memorial Sloan-Kettering Cancer Center in 2010. In Southeast Asia, the early proponents of CESM are Taiwan and Thailand in 2012.
The advantages of using CESM are that it is similar in diagnostic performance with Magnetic Resonance Imaging. Two studies have shown the similarity of Contrast-enhanced Spectral Mammography to MRI. Jochelson et al., found that CESM and MRI have equal sensitivity (96%) while Fallenberg et al., found CESM to have 100 % sensitivity compared with 97% sensitivity for MRI.[10],[11] This was further corroborated by Lee-Felker et al., who also had similar sensitivity of CESM to MRI (94% vs. 99%) as well as having a significantly higher PPV (Positive Predictive Value) of 93% compared to 60% of MRI.[12]
It can be used in patients with contraindications to doing MRI such as claustrophobia and/or averse to gadolinium contrast.
Furthermore, a recent study by Patel et al., showed that CESM had a reduced exam time of 7-10 minutes compared to MRI with 30-60 minutes as well as reduced staff time of 25 minutes compared to 60 minutes.[13]
The disadvantages include its contraindication for use in patients with abnormal renal function or if they have a known reaction to iodine contrast. Furthermore, it is not advised for pregnant or lactating women or those who are diagnosed with hyperthyroidism.
How is it performed?
Before a CESM exam is initiated, a thorough history is elicited from the patient with emphasis on allergy history and previous or known allergy to iodine contrast media. The creatinine level is obtained at least a week before the scheduled exam. Ideally, for premenopausal women, the timing of the exam should coincide with Days 7-14 of her menses, to reduce background parenchymal enhancement.
An IV is inserted into the forearm or antecubital vein and a power injector at a rate of 3 ml/s infuses iodinated contrast agent. The volume is typically calculated at 1.5-ml/kg bodyweight. The iodine concentration ranges from 300 mg/ml to 370 mg/ml.
After infusion of the contrast media, let 2 minutes pass before positioning the patient for the standard mammographic views (Mediolateral Oblique and Craniocaudal views) of each breast. For each projection, two energy pairs – low energy and high energy are generated in a single compression. Severe acute reactions to contrast media occur in 4/10,000 (0.04%) patients.[14]
According to a study of Lalji et al., the low-energy CESM images are non-inferior to Full-Field Digital Mammography (FFDM) images with no significant differences in image quality, average glandular dose, and contrast detail.[15] The LE images therefore are equivalent to a standard mammogram. The additional radiation dose from the HE images in CESM was approximately 20% that of routine Full-Field Digital Mammography or the equivalent of 1 additional view.[10]
The core principle in functional imaging is based on tumor enhancement secondary to tumor neoangiogenesis. The process of tumor neoangiogenesis plays a central role in the growth and spread of tumors.[16] Tumor cells secrete vascular endothelial growth factor (VEGF), a potent angiogenesis activator that stimulates the formation and proliferation of endothelial cells.[17] The newly grown vessels are immature and differ from normal capillaries. They are tortuous and irregular, resulting in poorly efficient perfusion, they are leaky (especially to macromolecules), and they are independent of the normal mechanisms of regulation of the capillary blood flow. Hemodynamic characteristics of immature neovessels can be conservatively assessed by dynamic contrast-enhanced magnetic resonance imaging or computed tomography. Tissue enhancement depends on arterial input function, kinetics of distribution of blood into the capillary bed, leakage across the capillary walls, and volume of the interstitial space.[16]
In our current setting, the detection of breast cancer mainly utilizes analog film mammography, conventional digital mammography, digital breast Tomosynthesis (DBT/2D+3D Mammography) and breast ultrasound, which are based on morphologic (anatomic) information, as opposed to MRI (Magnetic Resonance Imaging) which gives functional information. MRI, however, is limited in its use in our local setting because of its limited availability and the cost is prohibitive. The sensitivity of CESM was found to be high (98%), underscoring its potential to rival the diagnostic performance of MRI, but with added advantages of improved accessibility and lower cost.[18] In women with dense breasts, Cheung et al., demonstrated that CESM is superior to mammography in both sensitivity and specificity with improvement from 71.5% to 92.7% and 51.8% to 67.9%, respectively.[19]
Akin to the improvements and technological advances made in the field of Pathology, namely, with the development of novel molecular characterization of breast cancer with cellular markers, functional-based imaging of breast cancer is poised to change the paradigm of current diagnostic practice.
Dual-energy Contrast-enhanced Spectral Mammography may provide added value in determining the microcalcifications that show enhancement with a diagnosis favorable to cancer or a lack thereof as virtually diagnostic for non-malignant or noninvasive subgroup of cancers.[20] Likewise, its ability to identify multifocal or multicentric disease enables it to adequately and simultaneously stage both breasts[21] for better pre-biopsy planning.
Local Experience
The first institute to acquire Contrast-enhanced Spectral Mammography is Health Cube Advanced Medical Imaging Unit in March 2016. It was initially utilized as part of the surveillance monitoring of patients with either mastectomy or post-breast conservation surgery and is currently integrated into the diagnostic workflow in the work-up of patients with clinically suspicious findings, particularly those who have dense breasts.
The two cases included in this case series showcase the ability of CESM to image cancer whether in dense or non-dense breasts. The first case illustrates cancer without background parenchymal enhancement in a non-dense breast while the second case shows two (2) foci of cancer in a patient with dense breasts with additional background parenchymal enhancement.
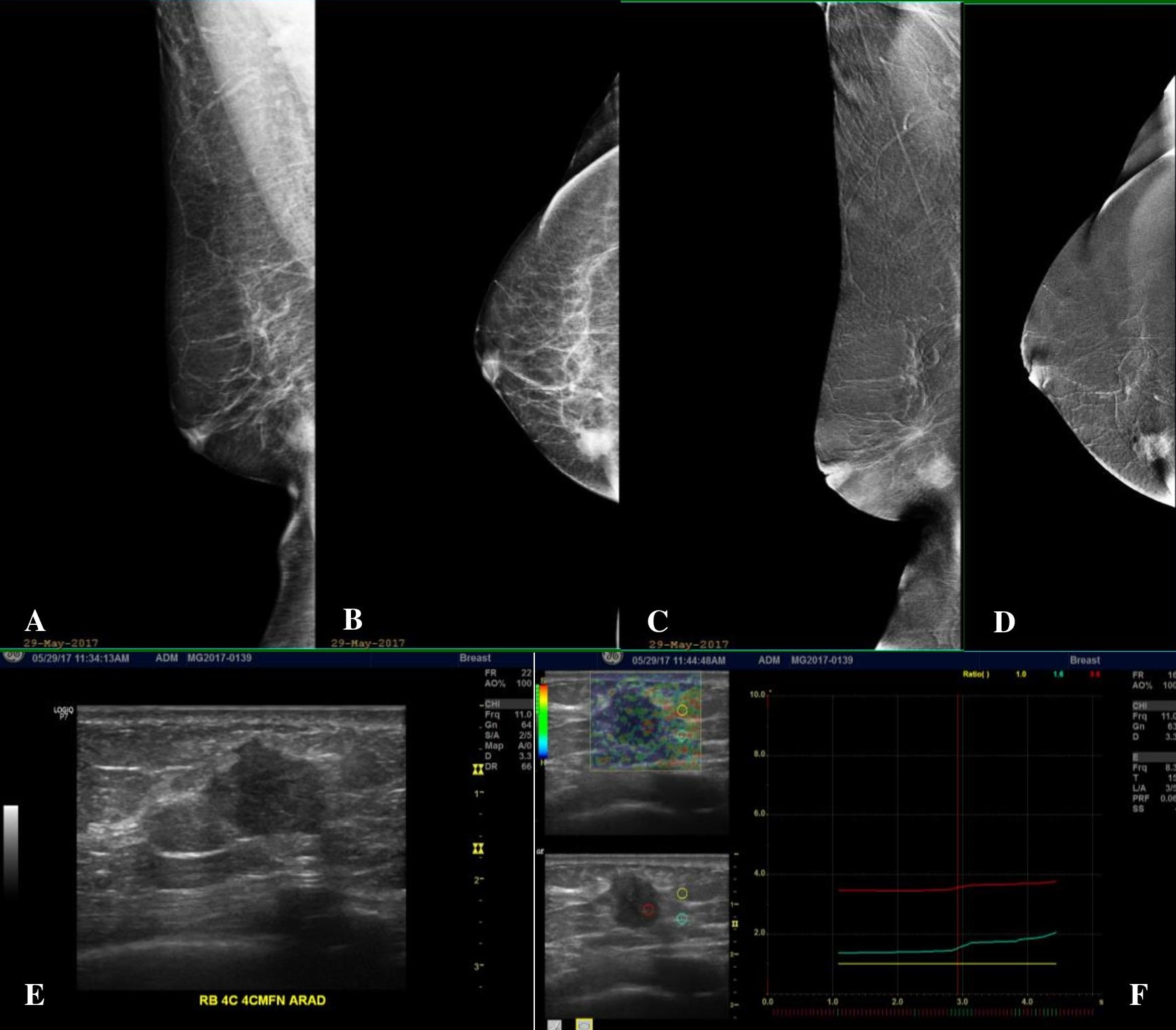
Figure 1. A 63-year-old post-menopausal female with a newly palpable right breast mass with no family history of breast cancer. (A, B) Low-energy (LE) Mediolateral Oblique (MLO) and Craniocaudal (CC) views of the right breast, which has a fatty breast composition, showing an irregular mass of high density at the lower inner quadrant. (C, D) Subtracted images (SI) in MLO and CC projections show avid enhancement of the right lower inner quadrant breast mass. No other abnormal enhancing lesions are demonstrated. The background parenchyma also shows no enhancement. (E) Ultrasound correlate of the said mass shows an irregular markedly hypoechoic solid mass. (F) Elastography (which is a measure of tissue stiffness) shows the mass to be significantly hard compared to the adjacent fat and glandular tissues.

Figure 2. Histopathologic features of the high-density mass seen in the right breast of the patient discloses invasive ductal carcinoma. (A) Seen are neoplastic ductal structures infiltrating the surrounding structures including the adjacent adipose tissue (H&E, 40x). (B) Other areas prominently show the formation of new blood vessels amidst clusters of tumor cells with associated extensive desmoplasia (H&E, 100x).
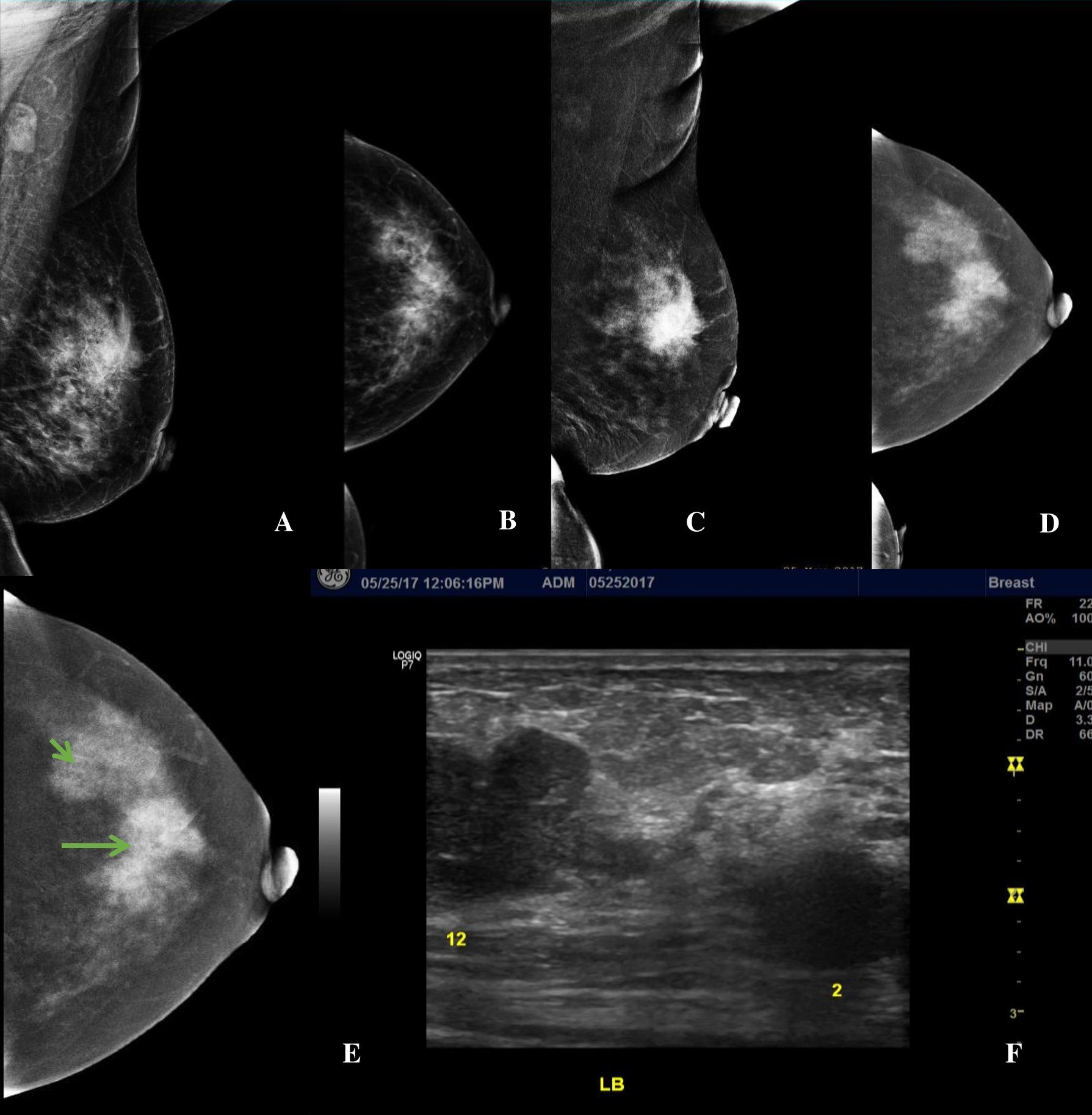
Figure 3. A 56-year-old premenopausal female presenting with a palpable left breast mass, Family history revealed she had two (2) sisters who had breast cancer in their 50’s. (A, B) LE MLO and CC views of the left breast showing heterogeneously dense breast composition. There is an irregular mass of increased density at the mid upper quadrant and a second equal density mass with partly obscured margins at the upper outer quadrant (C, D) Subtracted images (SI) in MLO and CC projections show an enhancing irregular mass at the mid upper left breast and shows the second enhancing mass with lobulations at the upper outer quadrant on a background of moderate parenchymal enhancement. (E, F) SI Image of the left CC view and the correlate ultrasound image showing multifocal breast lesions at the 12 o’clock (orange arrow) and 2 o’clock (orange arrowhead) positions.
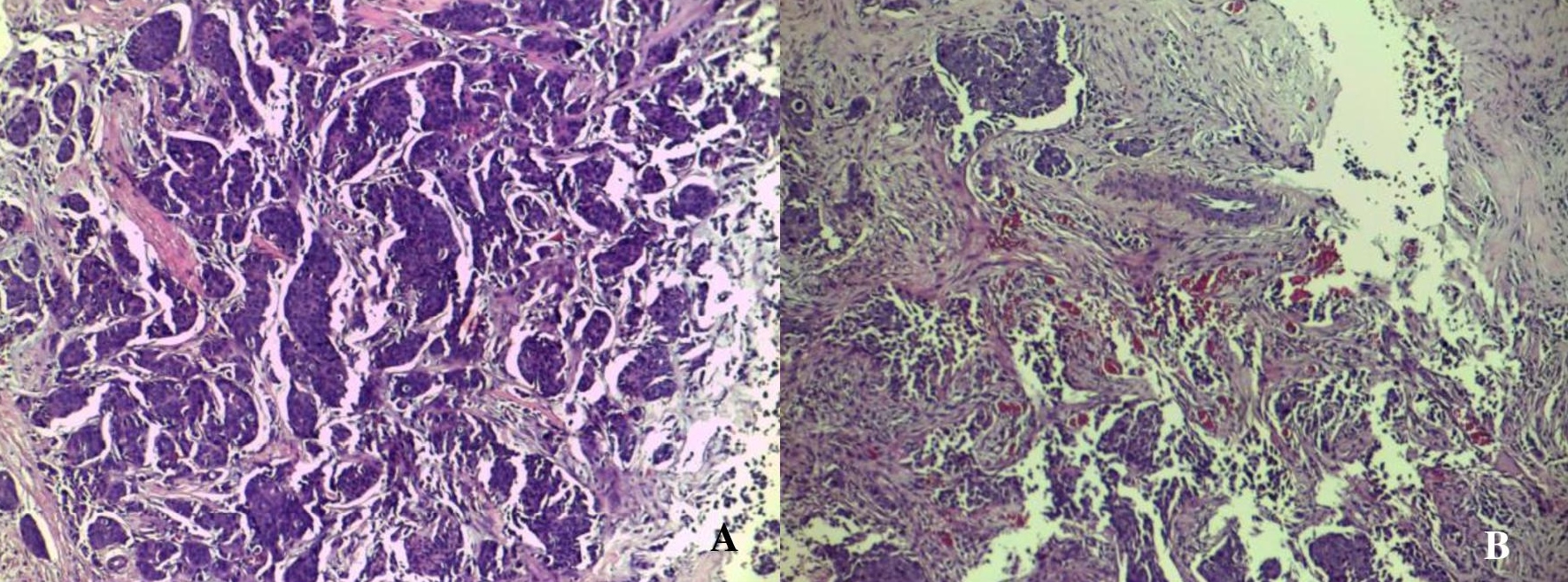
Figure 4. Microscopic appearances of the core biopsy taken from the enhancing left breast mass identified in the patient showing invasive ductal carcinoma. The tumor is very cellular and is composed of neoplastic cells disposed in tongues, cords and groups with attempts to form ducts (A). Note the pronounced desmoplastic reaction, which adds to the density seen radiographically. Some areas of the lesion reveal an abundance of well- and newly-formed blood vessels (B) (H&E, 100x).
The trend towards molecular and functional-based diagnosis of breast pathology will continue and potentially become the standard of care in the near future, making it possible for a tailored and targeted approach in the improved management of breast cancer.
Contrast-enhanced spectral mammography stands to be a viable and practical diagnostic imaging modality that can contribute to increased cancer detection rate and improve breast cancer care in our country.
The authors thank the following esteemed colleagues: Sherry L. Lee, MD, FPCS, FPSGS of Cardinal Santos Medical Center, and Timothy James Lam, MD of the University of the East Ramon Magsaysay Memorial Medical Center for the patient referrals and Paulo Giovanni L. Mendoza, MD, FPSP of Cardinal Santos Medical Center for his invaluable input on the histologic findings.
Patient consent was obtained before submission of the manuscript.
All authors certified fulfillment of ICMJE authorship criteria.
The authors declared no conflict of interest.
None.
[1] Jochelson M. Advanced imaging techniques for the detection of breast cancer. Am Soc Clin Oncol Educ Book. 2012:65-9. PMID DOI.
[2] Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA. 2015;314(15):1615-34. PMID DOI.
[3] Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003:138(3):168-75. PMID.
[4] Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227-36. PMID DOI.
[5] Yaghjyan L, Colditz GA, Collins LC, et al. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J Natl Cancer Inst. 2011;103(15):1179-89. PMID PubMed Central DOI.
[6] del Carmen MG, Halpern EF, Kopans DB, et al. Mammographic breast density and race. AJR Am J Roentgenol. 2007;188(4):1147-50. PMID DOI.
[7] Lobbes MB, Lalji U, Houwers J, et al. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol. 2014;24(7):1668-76. PMID DOI.
[8] Chang CH, Nesbit DE, Fisher DR, et al. Computed tomographic mammography using a conventional body scanner. AJR Am J Roentgeol. 1982;138(3):553-8. PMID DOI.
[9] Lewin JM, Isaacs PK, Vance V, Larke FJ. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology. 2003;229(1):261-8. PMID DOI.
[10] Jochelson MS, Dershaw DD, Sung JS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology: 2013;266(3):743-51. PMID DOI.
[11] Fallenberg EM, Dromain C, Diekmann F, et al. Constrast-enhanced spectral mammography vs. MRI: initial results in the detection of breast cancer and assessment of tumor size. Eur Radiol. 2014;24(1):256-64. PMID DOI.
[12] Lee-Felker SA, Tekchandani L, Thomas M, et al. Newly diagnosed breast cancer: comparison of contrast-enhanced spectral mammography and breast MR imaging in the Evaluation of Extent of Disease. Radiology. 2017;285(2):389-400. PMID DOI.
[13] Patel BK, Gray RJ, Pockaj BA, Potential cost-savings of contrast enhanced digital mammography. AJR Am J Roentgenol. 2017;208(6):W231-7. PMID DOI.
[14] Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621-8. PMID DOI.
[15] Lalji UC, Jeukens CRLPN, Houben I, et al. Evaluation of low-energy contrast-enhanced spectral mammography images by comparing them to full-field digital mammography using EUREF image quality criteria. Eur Radiol. 2015:25(10):2813–20. PubMed Central DOI.
[16] Cuenod CA, Fournier L, Balvay D, Guinebretière JM. Tumor angiogenesis: pathophysiology and implications for contrast-enhanced MRI and CT assessment. Abdom Imaging. 2006;31(2):188-93. PMID DOI.
[17] Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359-70. PMID DOI.
[18] Tagliafico AS, Bignotti B, Rossi F, et al. Diagnostic performance of contrast-enhanced spectral mammography: systematic review and meta-analysis. Breast. 2016;28:13-9. PMID DOI.
[19] Cheung YC, Lin YC, Wan YL, et al. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind- reading analysis. Eur Radiol. 2014;24(10):2394-403. PMID DOI.
[20] Cheung YC, Yuan YH, Lin YC, Lo YF, Tsai HP, Ueng SH, et al. Dual-energy contrast-enhanced spectral mammography: enhancement analysis on BI-RADS 4 non-mass microcalcifications in screened women. PLOS ONE. 2016;11(9):e0162740. DOI.
[21] Bhimani C, Malta D, Roth RG, Liao L, Timney E, Brill K, Germaine P. Contrast-ehanced spectral mammography: technique, indications, and clinical applications. Acad Radiol 2017;24(1):84-8. PMID DOI.