The External Quality Assessment Scheme for Transfusion Transmissible Infections in the Philippines aims to raise the standards of quality testing for infectious diseases in blood units.
The National Blood Program lists more than 200 Blood Service Facilities (BSF) in the country in which 162 participated in the 2016 EQAS test event. These participants were given an EQAS panel composed the HVHT4320 serology program and MLRA415 malaria program. The panels should be treated by the participants as routine donor samples to simulate the actual laboratory process which allows the NRL and the participant to check and validate the entire blood unit screening process.
The results were submitted via an online informatics system and were analyzed by One World Accuracy Canada using the ISO 13528:2008 Robust Statistics method (Huber’s Method) to identify outliers. Qualitative results were evaluated and compared with the reference results of the NRL to which non-concordance would mark their results aberrant. The results of the test event showed a number of participants having aberrant results due to either random or systematic errors.
Data gathered from this EQAS test event are used to improve the processes of the blood service facility to ensure quality testing.
Key words: quality assurance, blood donor serology, transfusion transmissible infections, proficiency testing
External Quality Assessment is a crucial aspect of quality assurance in medical laboratories. The results generated from each test event continuously reflect the analytical quality of the measurements performed by the participating laboratory and the performance can also be compared with other laboratories using the same instrument or method.[1]
The External Quality Assessment Scheme (EQAS) for Transfusion Transmissible Infections (TTI) in the Philippines is a mandatory requirement for licensing of Blood Service Facilities whose category are Blood Centers and/or Blood Bank with Additional Functions[2] that aims to raise the standards on quality testing of blood units and assess each phase of testing to determine inter-laboratory comparison.
This activity intends to assess the quality of blood unit testing of blood service facilities in the Philippines for the EQAS 2016 test event.
The TTI EQAS test event consists of two panels, the HVHT4320 for blood donor serology, and the MLRA415 for malaria slide microscopy. The HVHT4320 consists of twenty (20) pooled plasma samples obtained from blood donors from different regions of the country. Each pooled sample was prepared by mixing similar volumes of at least two samples that had similar antibody and antigen profiles. All samples were subjected to filtration prior to aliquoting. The samples were aliquoted and their homogeneity confirmed. The serology profile for HIV, HBV, HCV, Syphilis of each sample were identified using a chemiluminescence assay (ChLIA), enzyme immunoassay (EIA), Rapid Plasma Reagin (RPR), Particle Agglutination (PA) and Western Blot (WB).
Program code MLRA415 consists of five (5) blood smears. The samples were obtained from Malaria patients in Palawan and prepared by the NRL for Malaria and other Parasites of the Research Institute for Tropical Medicine
ParticipantsThe Multimarker Blood Serology EQAS panel ID HVHT4320 and Malaria Microscopy EQAS Panel ID MLRA415 were distributed to 162 participants nationwide. These participants enrolled for the EQAS 2016 test event with a corresponding registration fee to cover expenses for the test event.
Majority of the participants were private institutions (43%) followed closely by government institutions (41%) and the remainder are from the Philippine Red Cross (16%). Figure 1 shows the distribution of participants by region.
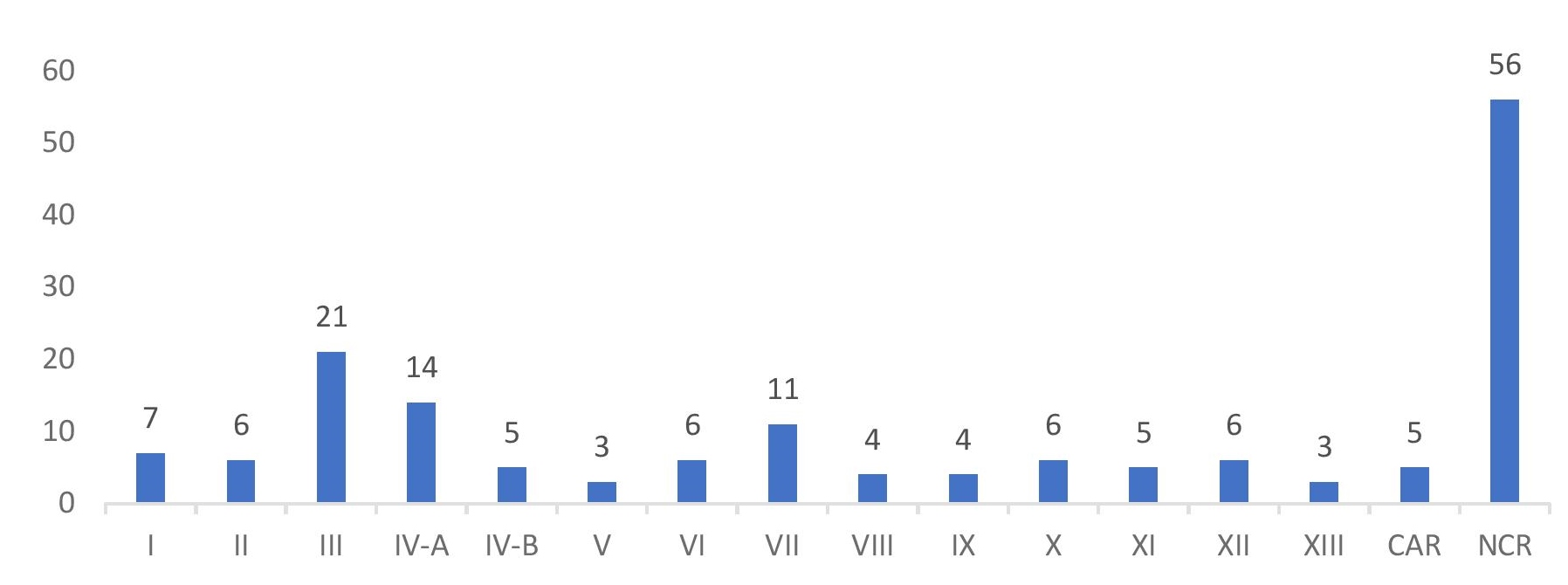
Figure 1. Regional distribution of participants.
Data Analysis
ISO 13528:2005 Robust Statistics method (Huber’s Method) was used to identify outlying results (numerical test results found to be statistically different from other test results reported by participants that tested the same sample in the same assay) for the created peer groups. A peer group is defined as a set of laboratories that utilize the same test format and assay test kit for screening TTI. The said method uses the mean as an estimator and outlying test results were removed from statistical calculation. Qualitative results of the BSF were compared with the qualitative reference results of the NRL Discrepancy between the two results would mark a result aberrant.
Majority of the participants used the ChLIA platform (48.77%) in testing the panels followed by EIA (29.63%). 6.79% used a combination of ChLIA and EIA, 1.85% used rapid test kits alone, and 12.96% used a combination of either ChLIA, EIA, Rapid Test Kits (RDT), Rapid Plasma Reagin (RPR), and Particle Agglutination (PA).
4.94% of the total participants had data entry errors or clerical errors (e.g. reactive test results were interpreted as negative or vice versa).
Of the 162 participants, 29.01% reported aberrant results for the HVHT4320 serology panel. A total of 13,374 results were reported by the participants and 91 (0.68%) were marked as aberrant. From the aberrant results, 47 (51.65%) were reported as false positive, 27 (29.67%) were reported as false negative, and 17 (18.68%) were reported as inconclusive. From the 27 false negative results, 11 (40.74%) were due to clerical errors.
Distribution of aberrant results by platform and analyte for the initial panel is shown in Table 1. These aberrant results were either due to data entry errors, sample mix-up or sample carry-over (particularly where an instrument was used in assay set-up).
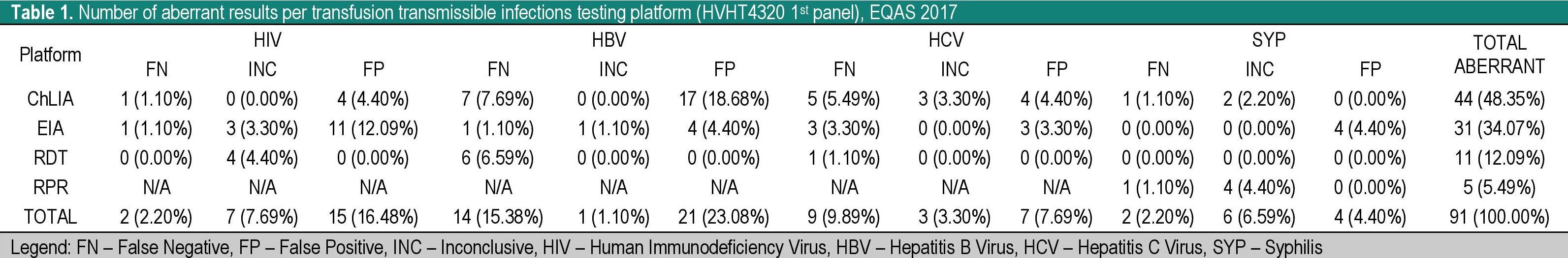
Table 1. Number of aberrant results per transfusion transmissible infections testing platform (HVHT4320 1st panel), EQAS 2017
The following criteria must be met for a participant to be classified as an unsatisfactory performer in the HVHT4320 initial panel: (a) at least one false negative result; (b) at least twenty percent (20%) false positive results. In accordance with these criteria, corresponding participants were given an investigation checklist to assist them in identifying errors and make the necessary corrective actions and/or troubleshooting methods. A 2nd set of the HVHT4320 panel were given to the participants for retesting if the identified unsatisfactory performance was due to a testing error. Participants with aberrant results due to transcription errors were only given an investigation/troubleshooting checklist and a written recommendation. Eight (8) participants were identified with transcription errors which they have recognized in the given investigation checklist. Ten (10) participants were given a second set of samples wherein one had reported a false negative result.
For the MLRA415 panel, 33% of the participants reported aberrant results with 8.64% reporting false positive results and 30.25% reporting false negative results.
Figure 2 shows the distribution of grades of the participants. They have been evaluated and graded as follows: • Excellent – 100% acceptable results on the initial panel (all final results were correctly identified in comparison with the reference results); • Very Satisfactory – Less than 100% acceptable results on the initial panel without being given a second panel for retesting. • Satisfactory – 100% acceptable results on retesting of the second panel; or had an aberrant result in the initial panel due to a clerical error, given that the participant was able to identify this error through the EQAS investigation checklist. • Poor – Participant did not follow minimum requirements of testing as per DOH Circular No. 2013-0132 or less than 100% acceptable results on retesting of the second panel; or had an aberrant result in the initial panel due to a clerical error which the participant had failed to identify in the EQAS investigation checklist.

Figure 2. Distribution of grades for the EQAS 2016 test event.
The TTI EQAS is a valuable management tool aimed to improve the efficiency and service of a laboratory. While this event has shown a number of participants failing, the results should be used as an opportunity to compare their activities and remodel their current practices based on what they would learn. A strong commitment from top-level managers of these participants is essential to improve these processes.
The participating laboratory should be responsible in reviewing their EQAS report and in discussing it to the people involved in the process since this is an opportunity for improvement by way of a corrective action. The analyzed data can improve the quality of results from the participants as this can be used to as evidence to introduce or improve the quality assurance of the laboratory.[3] An increase in the number of EQAS test events within a year would be of value in the improvement of the BSF processes.
The authors thank the TTI-NRL staff, Dr. Catherine Masangkay and Dr. Socorro Lupisan of the Research Institute for Tropical Medicine (RITM), the Health Facility Development Bureau, the Health Facility Services and Regulatory Bureau, and the National Voluntary Blood Services Program of the Department of Health, the National Council for Blood Services–Technical Working Group, the Department of Parasitology (RITM), the Philippine Red Cross – National Blood Center (Port Area), the Asian Hospital and Medical Center, and OneWorld Accuracy – Canada.
We also extend our thanks to all the participating Blood Service Facilities for their support.
All authors certified fulfillment of ICMJE authorship criteria.
The authors declared no conflict of interest.
None.
[1] Kristensen GB. Flowchart for Handling Deviating Results from External Quality Assessment. Int J Health Care Qual Assur. 2010; 20(1):37.
[2] Department of Health. DOH Department Memorandum No. 2009-0086B: Amendment to Department Memorandum No. 2009-0086-A entitled, “Implementation of external quality assessment program as regulatory requirement for licensing of clinical laboratories.” 8 September 2014. Retrieved from http://ttinrl.com/wp-content/uploads/2014/11/DM-2009-0086-B.pdf.
[3] World Health Organization. WHO manual for organizing a national external quality assessment programme for health laboratories and other testing sites. Geneva, Switzerland, 2016. Available from: http://www.who.int/hiv/pub/toolkits/manual-external-quality-assessment-testing/en/.