DOI: https://doi.org/10.21141/PJP.2017.004
Background. Sepsis is difficult to diagnose clinically because the signs and symptoms are non-specific. Blood culture is the gold standard, but it has low sensitivity and it takes at least 24-48 hours before results are released. Cell population data such as mean neutrophil volume (MNV) has recently has been shown to be significantly increased in septic patients both with high WBC and normal/low WBC count.
Objective. The aim of the present study was to conduct a meta-analysis of published papers on the accuracy of MNV in diagnosing sepsis relative to blood culture.
Methodology. Electronic databases including PubMed/Medline, Elsevier/Scopus, and Google Scholar were reviewed. Papers that were not retrieved in full text and papers that do not have data on MNV were excluded. The sensitivity and specificity were pooled, and the area under the receiver operating characteristic curve (AUROC) is computed.
Results. Seven studies including 994 participants were included in the meta-analysis. With a mean cut-off value of 153.15 fL [149.1315, 157.1685], the pooled sensitivity and specificity were 0.82 [0.71, 0.89], and 0.78 [0.68, 0.86] respectively. The AUROC is 0.87 [0.83-0.89].
Conclusion. MNV is a potential indicator for sepsis with high specificity and sensitivity, with moderate to high test accuracy. GRADE evaluation indicated a moderate quality of evidence: despite the large effect size, there is a serious risk of bias and high heterogeneity between the included studies.
Key words: Mean Neutrophil Volume, sepsis, accuracy, blood culture
Sepsis is difficult to diagnose because the signs and symptoms are non-specific. Clinical and laboratory findings are used for the diagnosis, and the gold standard is blood culture.[1] Blood culture has its shortcomings, such as low sensitivity, the need for sterile collection techniques to avoid contamination, and false positivity. There is a delay of at least a 24-48 hours before results of blood culture are available.[2] Early diagnosis and appropriate management are critical to reducing mortality.[3] Early diagnosis may be more effective in overall cost containment and outcome than a more specific but late diagnosis.[4] Therefore, a rapid, accurate, and cost-effective test is needed.[3]
However, early diagnosis of sepsis is difficult because the signs and symptoms of sepsis such as fever, are nonspecific and may be blunted or absent.[5] Peripheral blood smears can also yield important diagnostic information by identifying characteristic morphologic changes seen in reactive neutrophils.[4] Characteristic morphological changes regarding the size of the cell, the density of the nucleus, number of nuclear lobes, along with the presence of toxic granules, vacuolization and occasional Döhle bodies are evident in sepsis.[6] However, this approach is labor-intensive and time-consuming because it requires manual examination and an experienced medical technologist and pathologist. Furthermore, the results are subjective because they depend on human interpretation, and only a few hundred cells can be analyzed for any given sample.[4]
Reactive segmented neutrophils tend to be larger and have lower nuclear complexity than their normal “resting” counterparts. This could be analyzed quantitatively by using an automated hematology analyzer with volume, conductivity, and scatter (VCS) technology.[7] The automated hematology analyzer with VCS technology can determine the traditional parameters, such as total white blood cell and differential counts, and also determine the intrinsic biophysical properties of over 8000 leukocytes as well as measure the degree of cell size variation. This is analogous to the microscopic evaluation of a peripheral blood smear but uses the most modern technology to refine the output. These measurements of cellular morphological properties are known as cell population data (CPD).[8] VCS technology is exclusive for Beckman-Coulter hematology analyzers, but other manufacturers may develop future models that generate cell population data as well.
Previous studies demonstrated that CPD such as mean neutrophil volume (MNV), measured in femtoliters (fL), and neutrophil volume distribution width (NDW), measured in fL, are significantly increased in septic patients both with high WBC and those with normal or low WBC counts.[9] The MNV and/ or NDW show superior sensitivity and specificity for predicting sepsis when compared with WBC, neutrophil percentage, band counts, C-reactive protein, or procalcitonin, proving to be new and promising indicators for the diagnosis of early sepsis.[3]
In this study, we performed a meta-analysis to evaluate the accuracy of mean neutrophil volume in diagnosing sepsis. Specifically, we aimed to: (a) determine the pooled sensitivity and specificity of MNV in detecting sepsis, and to (b) determine the area under the curve or diagnostic accuracy.
The data of this manuscript came from electronic databases and previous studies. Thus, it is not applicable to receive an ethics committee approval or follow the Declaration of Helsinki, and there is no need to get informed consent of patients.
Search strategyElectronic databases including PubMed/Medline, Elsevier/Scopus, and Google Scholar were reviewed as of October 20, 2016, to select relevant studies on sepsis and mean neutrophil volume. Search terms ("Mean Neutrophil Volume" orr MNV) AND (sepsis or infection) with limits: Published from 2000 to present, Human, English, were used for the initial screening. However, initial results showed MNV as murine norovirus. Thus another search was made with search terms ("Mean Neutrophil Volume" or MNV NOT murine) AND (sepsis or infection) with limits: Human, English.
Inclusion and exclusion criteriaStudies from the search were screened accordingly. We asked help from the Medical Library Librarian to retrieve papers without free access. The studies were included if they met the following criteria: (a) disease of interest is sepsis, (b) index test of MNV for diagnosis of sepsis, and, (c) reference test of positive blood culture as a criterion for inclusion in the sepsis group. Papers that were not retrieved in full text and papers that do not have the sensitivity and specificity of the MNV were excluded.
Data extractionUsing an electronic spreadsheet, the investigators extracted the following data – name of the first author, publication year, study region, age group, the number of samples for the sepsis and healthy groups, true positive, false positive, false negative, true negative, and cut-off points.
Risk of bias in individual studiesIndividual studies were critically appraised based on QUADAS 2 assessment. Publication bias is evaluated by Deek’s funnel test.
Statistical analysisThe statistical analysis was performed using STATA 13. In pooling the sensitivity and specificity, a bivariate mixed-effects regression framework was used because of the assumed heterogeneity in the study characteristics. This was evaluated by Q statistic.[10] A p-value <0.05 considered statistically significant heterogeneity is present. Multiple univariable bivariate meta-regression models were used as an exploratory analysis of threshold-related heterogeneity.[11]
In the current study, we conducted a literature search published between the years 2000 and 2016 to identify studies relevant to investigating the utility of mean neutrophil volume in the diagnosis of sepsis. We searched publications in PubMed/ Medline, Elsevier/Scopus and Google Scholar using the search terms ("Mean Neutrophil Volume" OR MNV NOT norovirus) AND (sepsis or infection), with limits: Human, and English; the manuscripts published were evaluated.
A total of 75 results were evaluated. The twenty-six duplications were removed. After investigating all titles and abstracts from these articles, 12 studies were taken into consideration.[1],[2],[4]-[9],[12][13][14][15] Figure 1 describes the selection process done following the aforementioned inclusion and exclusion criteria. A total of seven studies were included in the meta-analysis.
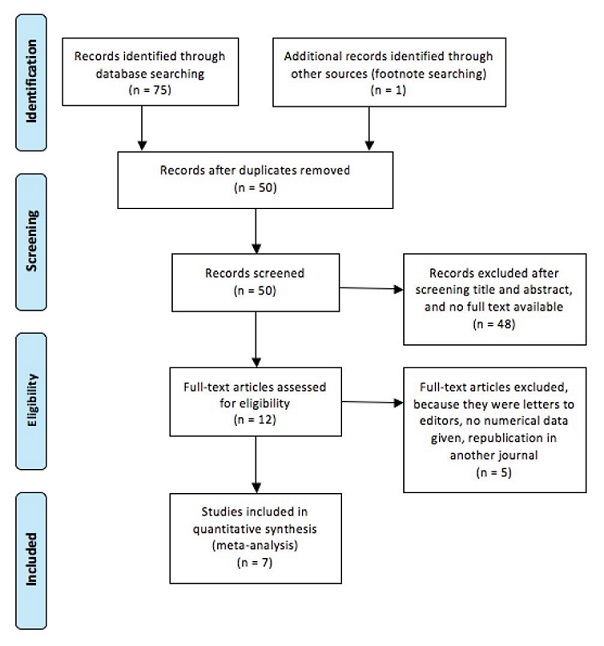
Figure 1. Process of study selection.
Study characteristics
The characteristics of the seven included studies are listed in Table 1. A total of 994 participants were involved in this metaanalysis. Five of the included studies were distributed in Asia while one study was conducted in Europe, and another in the USA. The patients who were positive for the blood culture test were included in the intervention (sepsis) group while healthy patients were classified as controls. Their MNV were assessed using the following hematological analyzers – LH750, LH780 and Dx800 Beckman Coulters (Fullerton, CA).
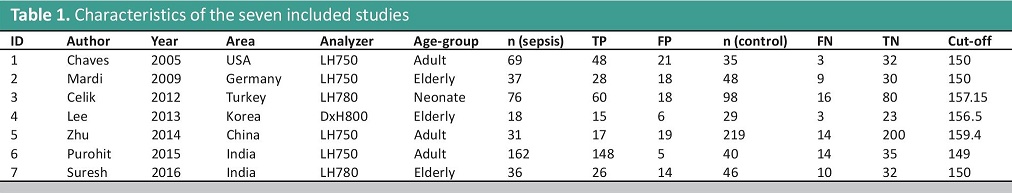
Table 1. Characteristics of the seven included studies
The included studies are heterogeneous based on nationality, analyzer used, and age-group. This necessitates a random effects model in pooling the sensitivity and specificity of the included studies. However, subgroup analysis cannot be made, because there is only one study in the following theoretical subgroups: DxH800 analyzer and Neonates.
Risk of bias within studiesThe risk of bias is serious because the included studies utilized a case-control design, and the index test cut-off used was optimized. The summary of the risk of bias is shown in Tables 2 and 3, and it was determined to have a serious risk of selection bias.
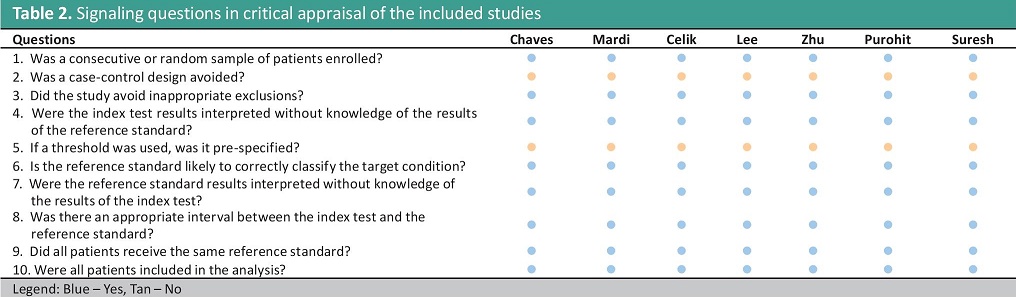
Table 2. Signaling questions in critical appraisal of the included studies
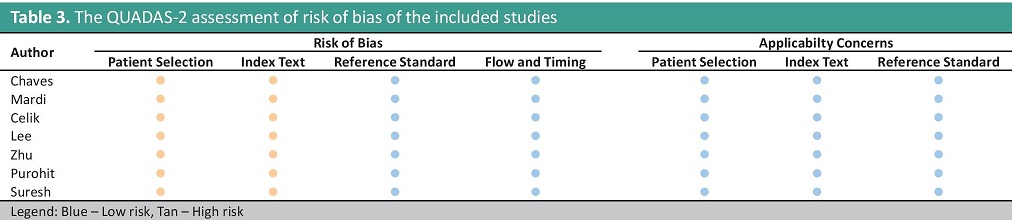
Table 3. The QUADAS-2 assessment of risk of bias of the included studies
Synthesis of results for the summary ROC analysis
The forest plot of the meta-analysis is seen in Figure 2. The calculated summary performance estimates and their corresponding 95% CIs are as follows: Sensitivity = 0.82 [0.71, 0.89], Specificity = 0.78 [0.68, 0.86], Positive LR = 3.7 [2.6, 5.4], Negative LR = 0.23 [0.15, 0.37] and Diagnostic Odds Ratio = 16 (9, 30). High heterogeneity was observed for the first two estimates – Q=33.78 (p<0.01) and Q=44.09 (p<0.01) respectively. To explain this, metaregression was made. It was found that the proportion of heterogeneity likely due to threshold effect was 27%.
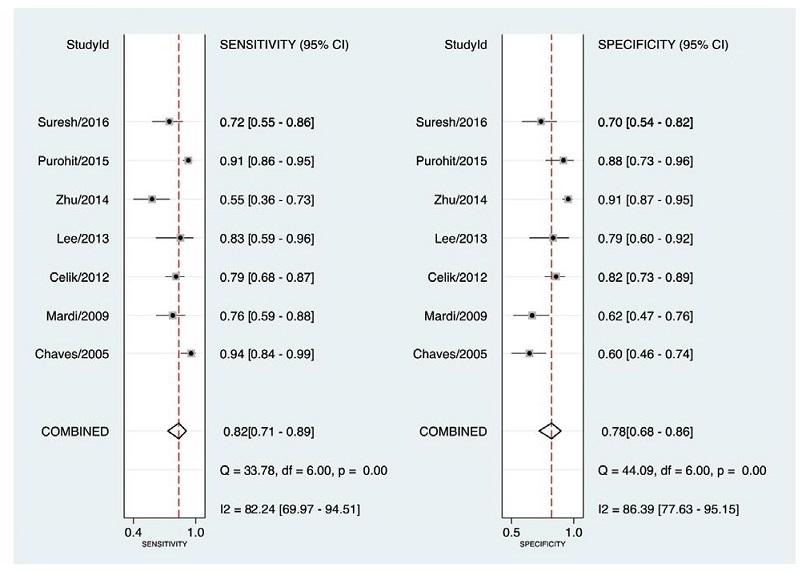
Figure 2. Forest plot showing study-specific and mean sensitivity and specificity with corresponding heterogeneity statistics.
The summary ROC curve is displayed along with the observed study data in Figure 3. The dashed line around the summary point estimate represents the 95% confidence region. The area under the curve (AUROC), serves as a global measure of test performance. The AUROC is the average TPR over the entire range of FPR values. The calculated AUROC was 0.87 [0.83-0.89] with high heterogeneity, Q=27.54 (p<0.001).
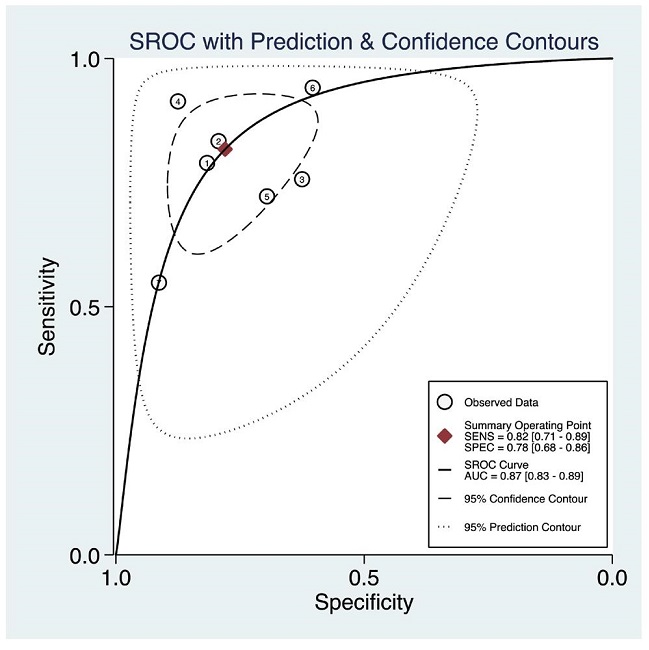
Figure 3. Summary ROC curve with confidence and prediction regions around mean operating sensitivity and specificity point.
The Deek’s Funnel Plot Asymmetry Test was conducted to explore possible publication bias. The studies appear to cluster around the regression line (Figure 4). The calculated p-value was also less than alpha =0.05 which suggests a low likelihood of publication bias.
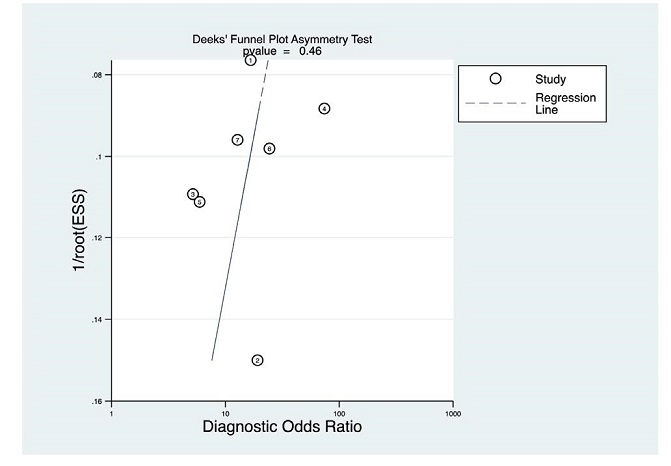
Figure 4. Deek’s Funnel Plot Asymmetry Test.
MNV is not routinely reported in complete blood count. Most clinicians rely on the result of blood culture test, which could take at least 24-48 hours. This meta-analysis explores the accuracy of MNV in detecting sepsis. The mean sensitivity and specificity were 0.82 [0.71, 0.89] and 0.78 [0.68, 0.86] respectively. This means 82% of those with sepsis have elevated MNVs above the cut-off; and, 78% of those without sepsis have MNVs lower than the cut-off. The Likelihood Ratio Positive is computed to be 3.72 [2.57, 5.37]. This means that an elevated MNV above cut-off increases the probability of presence of sepsis by approximately 30%. The Likelihood Ratio Negative is computed to be 0.23 [0.15, 0.37]. This means that an MNV below the cut-off decreases the probability of presence of sepsis by approximately 30%.
However, high heterogeneity was observed for both parameters [Q=33.78 (p<0.01) and Q=44.09 (p<0.01)] and it was found that 27% of the variability is due to the variability in threshold (cutoff value). The mean cut-off value from the studies is 153.15 fL [149.1315, 157.1685]. Because of the heterogeneity due to the cutoff values, two cut-off points should be used to determine elevated and non-elevated MNV. Elevated MNV is values above 157 fL to be used to rule in sepsis; and an MNV below 150 fL is to be used to rule out sepsis. Indeterminate results within 150 to 157 fL will require blood culture testing to proceed.
The calculated AUROC was 0.87 [0.83-0.89]. This suggests moderate to high test accuracy. However, there is high heterogeneity, Q=27.54 (p<0.001) observed. This heterogeneity may be due to several factors such as age group, model of analyzer used, and nationality. The accuracy of the use of MNV in diagnosis sepsis may vary depending on age group, and nationality of patient, as well as model of analyzer used.
The quality of evidence was evaluated to be moderate (Table 4).
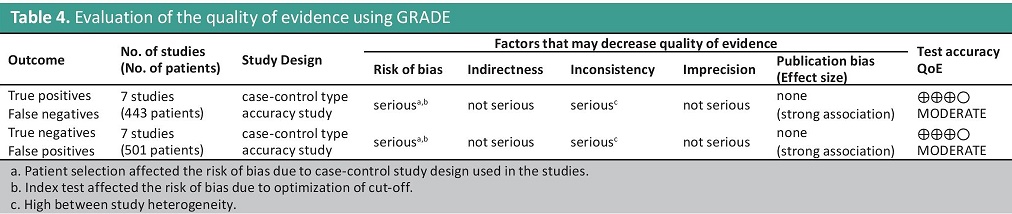
Table 4. Evaluation of the quality of evidence using GRADE
Limitations
The limitations of this study include: 1. no sub-group analysis was made because there is no sufficient number of studies for each subgroup; 2. a large proportion of heterogeneity was unaccounted for the Summary ROC Analysis; and, 3. included studies were limited to published studies. A subgroup analysis may account for the other proportions of heterogeneity. It may be due to the model of analyzer used, or variation in the cut-off points with regards to age group and nationality of patients. Since this study is limited to published studies, there might be unpublished studies that may fill up the subgroups needed to do a subgroup analysis.
In conclusion, MNV is a potential indicator for sepsis because of moderate to high test accuracy relative to blood culture. GRADE evaluation of quality of evidence, however, shows that there is a moderate level of the quality of evidence because despite the large effect size, the studies included were case-control, and there is high heterogeneity between studies. The authors recommend that subgroup analysis must be performed after attaining more studies per group, to explain the large proportion of unexplained heterogeneity.
The authors declared no conflicts of interest.
None.
[1] Celik IH, Demirel G, Aksoy HT, Erdeve O, Tuncer E, Biyikli Z, et al. Automated determination of neutrophil VCS parameters in diagnosis and treatment efficacy of neonatal sepsis. Pediatr Res. 2012;71(1):121-5. DOI.
[2] Suresh PK, Minal J, Rao PS, Ballal K, Sridevi HB, Padyana M. Volume conductivity and scatter parameters as an indicator of acute bacterial infections by the automated haematology analyser. J Clin Diagn Res. 2016;10(1):EC01-3. DOI.
[3] Charafeddine KM, Youssef AM, Mahfouz RA, Sarieddine DS, Daher RT. Comparison of neutrophil volume distribution width to C-reactive protein and procalcitonin as a proposed new marker of acute infection. Scand J Infect Dis. 2011;43(10):777- 84. DOI.
[4] Mardi D, Fwity B, Lobmann R, Ambrosch A. Mean cell volume of neutrophils and monocytes compared with C-reactive protein, interleukin-6 and white blood cell count for prediction of sepsis and nonsystemic bacterial infections. Int J Lab Hematol. 2010;32(4):410-8. DOI.
[5] Lee AJ, Kim SG. Mean cell volumes of neutrophils and monocytes are promising markers of sepsis in elderly patients. Blood Res. 2013;48(3):193-7. DOI.
[6] Purohit AH, Kumar P, Sharma S, Kapil A, Gupta A, Mukhopadhyay AK. Volume, conductivity, and scatter parameters as diagnostic aid to bacterial sepsis: a tertiary care experience. Indian J Pathol Microbiol. 2015;58(4):459-63. DOI.
[7] Chaves F, Tierno B, Xu D. Quantitative Determination of neutrophil VCS parameters by the coulter automated hematology analyzer: new and reliable indicators for acute bacterial infection. American J Clin Pathol. 2005;124(3):440- 4. DOI.
[8] Zhu Y, Cao X, Chen Y, Zhang K, Wang Y, Yuan K, et al. Neutrophil cell population data: useful indicators for postsurgical bacterial infection. Int J Lab Hematol. 2012;34(3):295-9. DOI.
[9] Zhu Y, Cao X, Zhang K, Xie W, Xu D, Zhong C. Delta Mean Neutrophil Volume (MNV) is comparable to procalcitonin for predicting postsurgical bacterial infection. J Clin Lab Anal. 2014;28(4):301-5. DOI.
[10] Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60. PubMed PubMed Central DOI.
[11] Dwamena BA. MIDAS: A program for eta-analytical integration of diagnostic accuracy studies in stata. Division of Nuclear Medicine, Department of Radiology, University of Michigan Medical School, Ann Arbor, Michigan; 2007.
[12] Abiramalatha T, Santhanam S, Mammen JJ, Rebekah G, Shabeer MP, Choudhury J, et. al. Utility of neutrophil volume conductivity scatter (VCS) parameter changes as sepsis screen in neonates. J Perinatol. 2016;36:733-8. DOI.
[13] Celik IH, Demirel G, Sukhachev D, Erdeve O, Dilmen U. Neutrophil volume, conductivity and scatter parameters with effective modeling of molecular activity statistical program gives better results in neonatal sepsis. Int J Lab Hematol. 2013;35(1):82-7. DOI.
[14] Çelik HT, Portakal O, Yiğit Ş, Hasçelik G, Korkmaz A, Yurdakök M. Efficacy of new leukocyte parameters versus serum C-reactive protein, procalcitonin, and interleukin-6 in the diagnosis of neonatal sepsis. Pediatr Int. 2016;58(2):119- 25. DOI.
[15] Bagdasaryan R, Zhou Z, Tierno B, Rosenman D, Xu D. Neutrophil VCS parameters are superior indicators for acute infection. Lab Hematol. 2007;13(1):12-6. PubMed DOI.