Objective. The study was undertaken to determine if commercially bottled purified water can be used as substitute instrument feed water for three (3) newborn screening immunoassays.
Methdology. A total of 294 control samples and 300 patient samples were included in this study. Accuracy and precision studies using control samples, and parallel testing using patient samples, were done to compare the use of clinical laboratory reagent water (CLRW) and commercially bottled purified water (CBPW) in the performance of automated time-resolved fluorescent immunoassay of thyroid stimulating hormone (TSH), 17α-OH-progesterone (17-OHP) and immunoreactive trypsinogen (IRT).
Results. The use of CBPW as instrument feed water for measurements of TSH, 17-OHP and IRT levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS has an acceptable accuracy and precision compared to using CLRW. The parallel testing using patient samples showed that, overall, the performance of using CBPW in automated time-resolved fluorescent immunoassay for TSH, 17-OHP, and IRT is acceptable, compared with using CLRW as instrument feed water.
Conclusion. Commercially bottled purified water can be used as substitute when setting up a laboratory water purification system is too expensive for a laboratory, or as back up to clinical laboratory reagent water when there is breakdown of the installed water purification system to be used as instrument feed water in automated time-resolved fluorescent immunoassay of TSH, 17-OHP and IRT in NBS using AutoDELFIA (Perkin-Elmer).
Key words: fluorescent antibody technique, immunoassay, neonatal screening, clinical laboratory reagent water
The Newborn Screening Study Group (NSSG) first conceptualized newborn screening (NBS) in the Philippines in 1996. The initial objectives of the Philippine Newborn Screening Project (PNBSP) were to establish the incidence data of six metabolic conditions – congenital hypothyroidism (CH), congenital adrenal hyperplasia (CAH), galactosemia (GAL), phenylketonuria (PKU), homocystinuria (HCY), and glucose-6-phosphate dehydrogenase (G6PD) deficiency, and to make recommendations for the adoption of newborn screening nationwide.[1] This project has been successful as a newborn screening bill was introduced and was signed into law in 2004 as Republic Act 9288 or Newborn Screening Act of 2004. This law requires that every newborn must be given access to NBS by the attending or assisting health practitioner.[2] Later on, there was discontinuation of screening for homocystinuria as a cost-cutting measure due to non-detection of cases, and inclusion of screening for maple syrup urine disease (MSUD) as part of the six basic screened disorders. Additional disorders were included in the expanded NBS (eNBS) in 2014. It included screening for cystic fibrosis (CF), biotinidase deficiency (BTND), hemoglobinopathies (HBP), amino acid metabolism disorders (AAD), acylcarnitine metabolism disorders (ACD), fatty acid oxidation disorders (FAO), and urea cycle disorders (UCD).
NBS for primary CH is done through determining the TSH level on a dried blood spot (DBS).[3] Infants with significantly elevated DBS TSH indicate risk for primary CH. An elevated serum TSH and a low serum FT4 confirm primary CH.[4] The 17α-OH-progesterone (17-OHP), a precursor of cortisol, is increased in the 2 most common types of CAH, i.e., 21- and 11β-hydroxylase deficiencies. Therefore, measuring the 17-OHP levels on DBS is a useful NBS method for the detection of CAH.[5] Infants with moderate to severe elevation of 17-OHP, and those who have mild elevation of 17-OHP and are low birth weight must undergo confirmatory tests with plasma 17-OHP, sodium, potassium, cortisol and glucose.[4] Screening for CF entails for initial measurement of IRT levels on DBS. An elevated IRT level signifies an increased risk of CF. The neonate then undergoes confirmatory testing wither by sweat test for chloride or a DNA test for CFTR mutations.[6] Since the initiation of the NBS in the Philippines, fluorescent immunoassay is the recommended laboratory method for NBS of CH, CAH, and CF.[1],[7],[8],[9]
Automated methods for detecting TSH and 17-OHP use solid phase time-resolved fluorescent immunoassay. Solid-phase methods utilize a washing step to separate the bound analyte, which is immobilized by the antibody attached to a solid support, from the unbound, which is washed away.[10] The presence of analyte is then detected by labeled indicator reagents using different techniques.[11] The TSH and IRT assays are based on a direct sandwich technique where two monoclonal antibodies recognize separate antigenic determinants on the TSH molecule. The fluorescence signal is proportional to the TSH concentration in the sample.[12] The 17-OHP assay, on the other hand, is based on the competitive binding of Europium-labeled 17-OHP, and 17-OHP in the sample to 17-OHP-specific antibodies. The fluorescence signal is inversely proportional to the 17-OHP concentration in the sample.[13] Excess, unbound labeled indicator reagents will be washed by another washing step before instrument reading.
Clinical laboratory reagent water (CLRW) should be pure enough to satisfy the requirements of most clinical laboratory testing. CLRW must have resistivity ≥ 10 MΩ.cm referenced to 25°C, total heterotrophic plate count <10 CFU/mL, total organic carbon <500 ng/g, and particulate content sizes of <0.22 μm. The CLRW are prepared though different available laboratory water purification systems. The automated method for time-resolved fluorescent immunoassay utilizes this CLRW as instrument feed water for internal washing, rinsing and dilution.
Commercially bottled purified water (CBPW) refers to water that is marketed for drinking.[14] Manufacture of CBPWs is regulated by law and should follow standards prior to commercial release for consumption. The Department of Health Administrative Order 10 series of 2017 requires the following physico-chemical and microbiologic standards: CBPW must have resistivity ≥0.2 MΩ.cm referenced to 25°C, total heterotrophic plate count <500 CFU/mL, and organic chemicals <0.0002 to 1mg/L, depending on the particular organic chemicals. There is no specified standard for total organic carbon and particulate content sizes.[15]
The objective of this study is to determine if CBPW, particularly Wilkins distilled water, can be used as substitute when setting up a laboratory water purification system is too expensive for a laboratory, or as back up to CLRW when there is breakdown of water purification systems, in the performance of automated time-resolved fluorescent immunoassay of TSH, 17-OHP, and IRT using AutoDELFIA (Perkin-Elmer) for NBS.
The study is limited to the evaluation of the above-mentioned analytes. These are the only analytes measured by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS in the Philippines.
A total of 294 control samples and 300 patient samples were included in this study. There were 61 low and 61 high TSH controls, 55 low and 55 high 17-OHP controls, 31 low and 31 high IRT controls, 100 TSH patient samples, 100 17-OHP patient samples, and 100 IRT patient samples. A single analyst did the sample preparation and operation of the instrument to control for possible inter-analyst variability in technique. A single reagent kit lot was used to control for possible inter-lot variability in the chemical reactions. Finally, a single automated time-resolved fluorescent immunoassay instrument using AutoDELFIA (Perkin-Elmer) was used to control for possible inter-machine variability in instrument performance. The type of water used, i.e. CLRW or CBPW (using Wilkins distilled water), was the experimental intervention for this study.
There were two phases of the study; first is the accuracy and precision studies. The accuracy of using CBPW in measuring control samples was compared to the reference method, i.e., using CLRW as instrument feed water. Mean and deviation were the statistic used to evaluate accuracy. A deviation less than |10%| and/or t-test between two independent means with t<〖Critical〗_t indicates an acceptable accuracy. The precision of CBPW compared to the precision of the CLRW in measuring control samples. Standard deviation (SD) and Coefficient of variation (CV) were the statistic used to evaluate accuracy. A CV less than |10%| and/or F-test between two variances with F<〖Critical〗_F indicates an acceptable precision.
The second part of the study is the parallel testing using patient samples. Bland-Altman analysis, Passing Bablok regression, and kappa statistic are used to evaluate the performance of using CBPW compared to using CLRW as instrument feed water. A bias less than |10%|, slope of between 0.90 to 1.10, linearity of 0.975 to 1.000, and kappa greater than 0.90 indicates an acceptable comparable performance of using CBPW in automated time-resolved fluorescent immunoassay.
Accuracy and Precision Studies
A total of 294 control samples were included in this phase. The results are summarized in Table 1. There were no significant mean differences in the measurements of the 17-OHP, TSH, and IRT levels of low and high control samples between CLRW and CBPW (t<〖Critical〗_t). The percent deviations of CBPW from CLRW were less than |10%| for all analytes and control levels. There was no significant difference in variances in the measurements of the 17-OHP, TSH, and IRT levels of low and high control samples between CLRW and CBPW (F<〖Critical〗_F), and the CV of CBPW were less than |10%| for all analytes and control levels. This indicates that using CBPW as instrument feed water for measurements of the 17-OHP, TSH, and IRT levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS has an acceptable accuracy and precision.
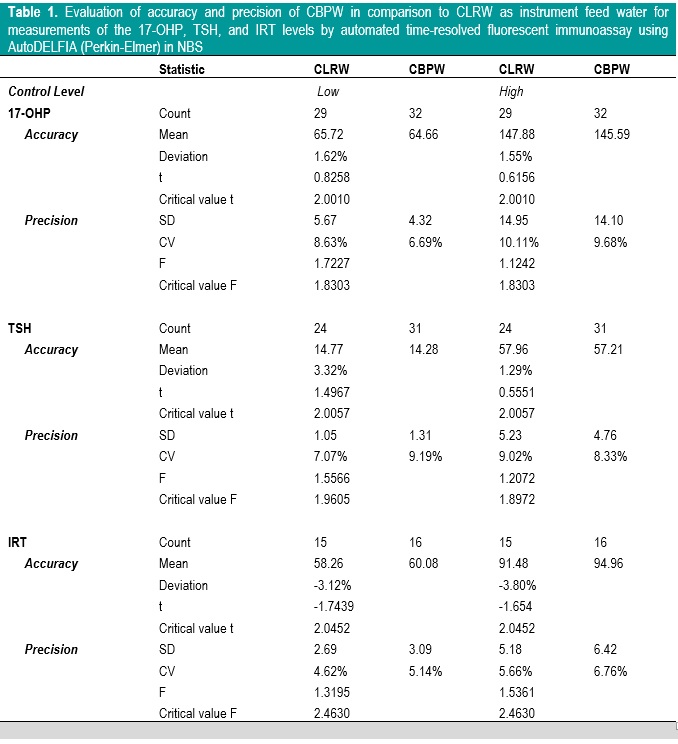
Table 1. Evaluation of accuracy and precision of CBPW in comparison to CLRW as instrument feed water for measurements of the 17-OHP, TSH, and IRT levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS
Parallel Testing of Patient Samples
A total of 300 patient samples were included in this phase. The results of Bland-Altman analysis and Passing Bablok regression analysis are summarized in Table 2. Generally, CBPW gives higher results than CLRW, with % bias less than |10%| for all analytes, and the slope and linearity are within 0.90 to 1.10 and 0.975 to 1.000, respectively.
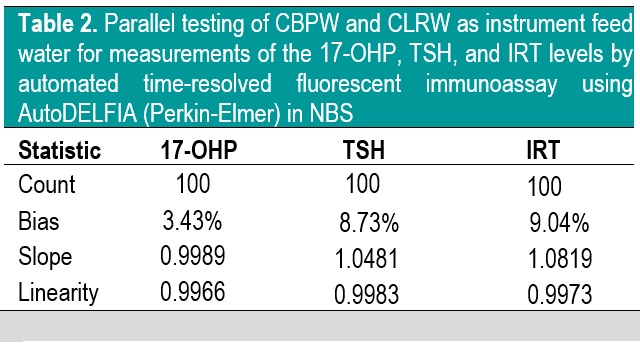
Table 2. Parallel testing of CBPW and CLRW as instrument feed water for measurements of the 17-OHP, TSH, and IRT levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS
Evaluation of the Bland-Altman plot and Passing Bablok regression line (Figures 1-6) shows that values near the cut-off values for 17-OHP, TSH, and IRT are within the agreement limits, and are close to the best fitted line, respectively. This may indicate that using CBPW as an alternative to CLRW would not misclassify the result of the screening test.
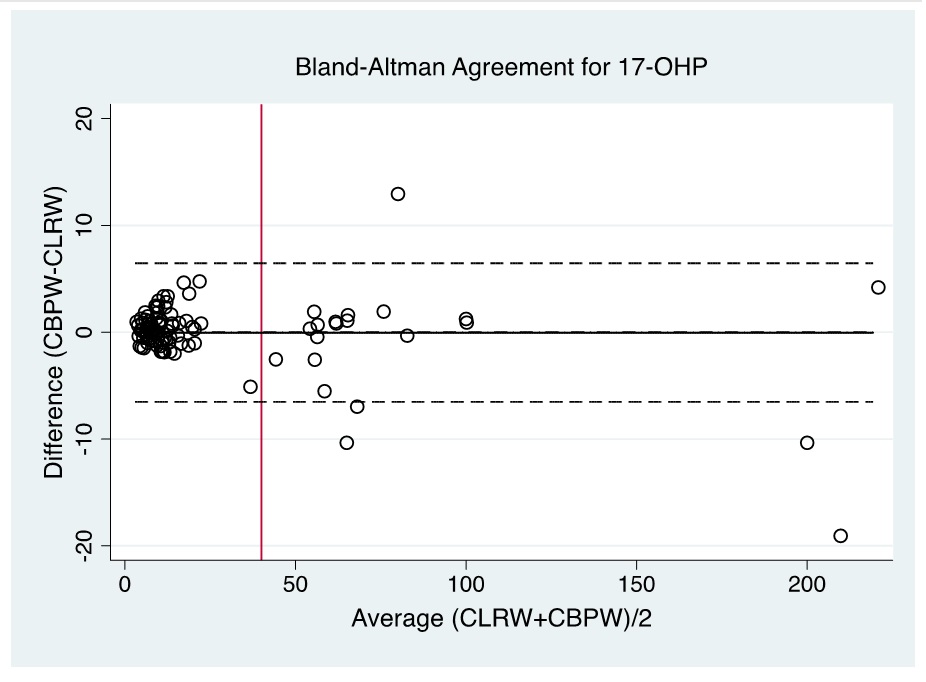
Figure 1. Bland-Altman plot of CBPW and CLRW as instrument feed water for measurements of the 17-OHP levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS. (Note: Red line indicates cut-off value).
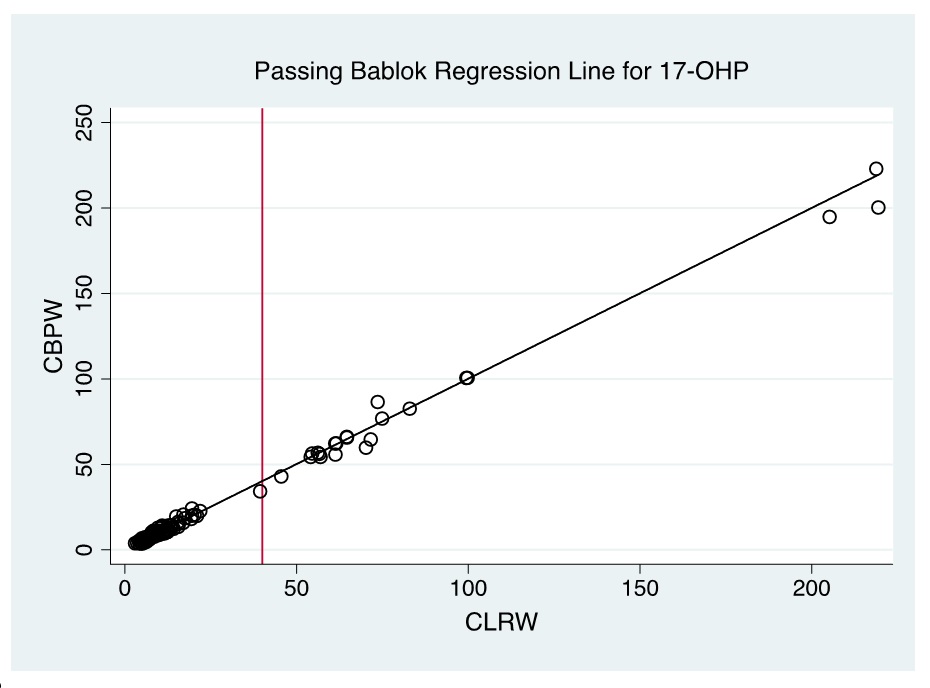
Figure 2. Passing Bablok regression line of CBPW and CLRW as instrument feed water for measurements of the 17-OHP levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS. (Note: Red line indicates cut-off value).
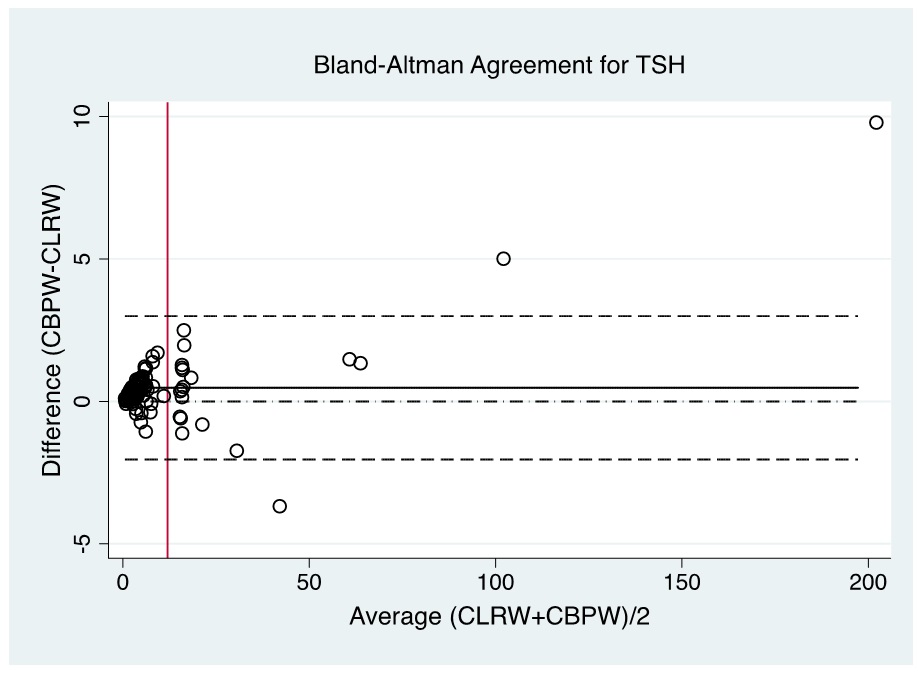
Figure 3. Bland-Altman plot of CBPW and CLRW as instrument feed water for measurements of the TSH levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS. (Note: Red line indicates cut-off value).
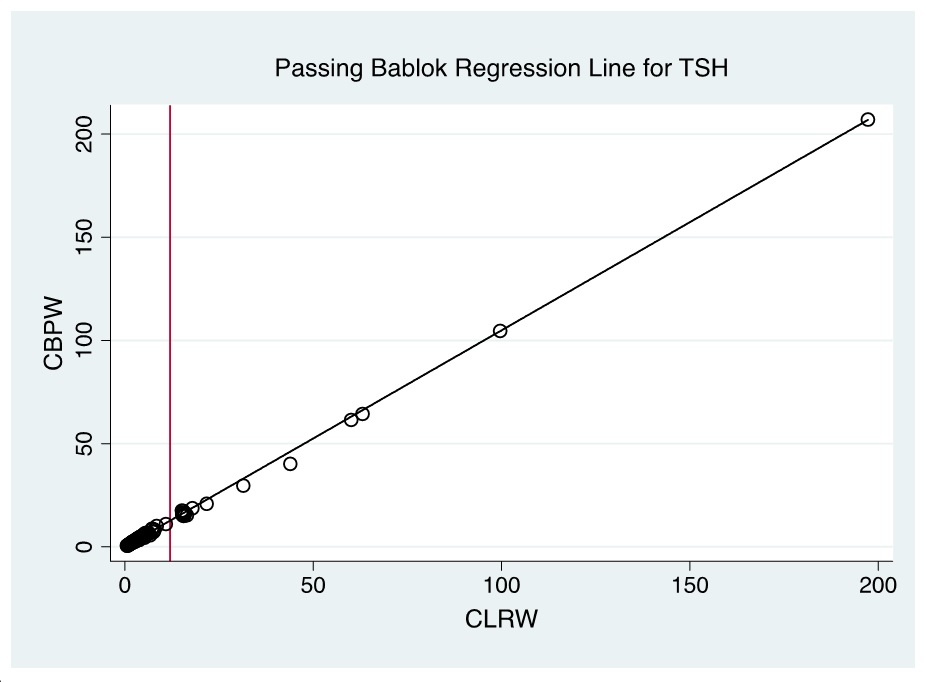
Figure 4. Passing Bablok regression line of CBPW and CLRW as instrument feed water for measurements of the TSH levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS. (Note: Red line indicates cut-off value).
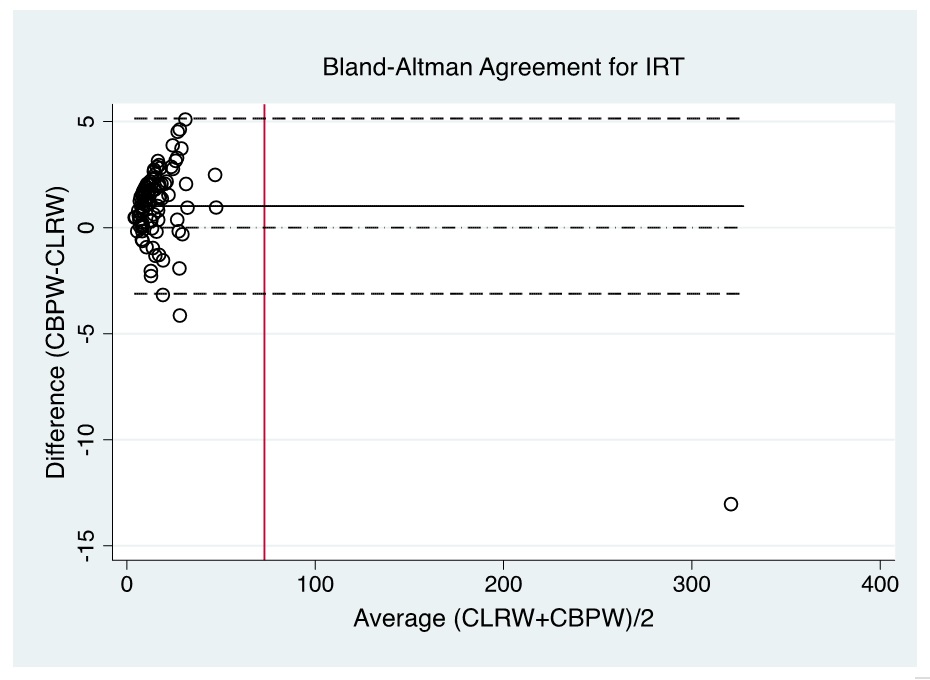
Figure 5. Bland-Altman plot of CBPW and CLRW as instrument feed water for measurements of the IRT levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS. (Note: Red line indicates cut-off value).
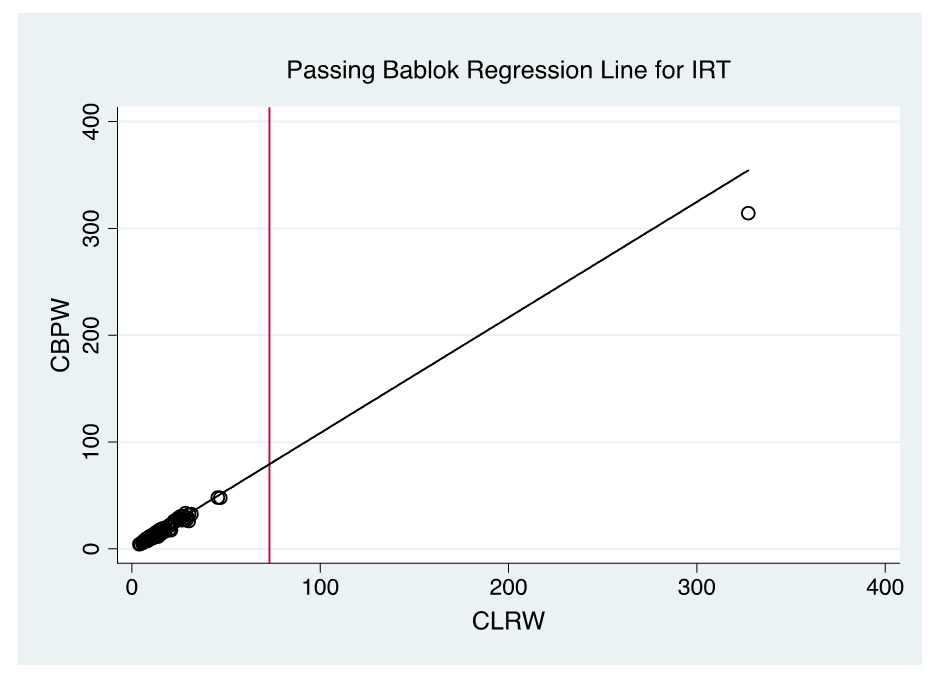
Figure 6. Passing Bablok regression line of CBPW and CLRW as instrument feed water for measurements of the IRT levels by automated time-resolved fluorescent immunoassay using AutoDELFIA (Perkin-Elmer) in NBS. (Note: Red line indicates cut-off value).
In NBS, it is the delineation of a positive versus a negative screen which is more critical than the actual quantitative value; therefore, the agreement in terms of kappa of the screening status of both methods is more significant to evaluate. Based on kappa statistic, there is a perfect level of agreement in the identification of positive screen between CBPW and CLRW (Tables 3-5).
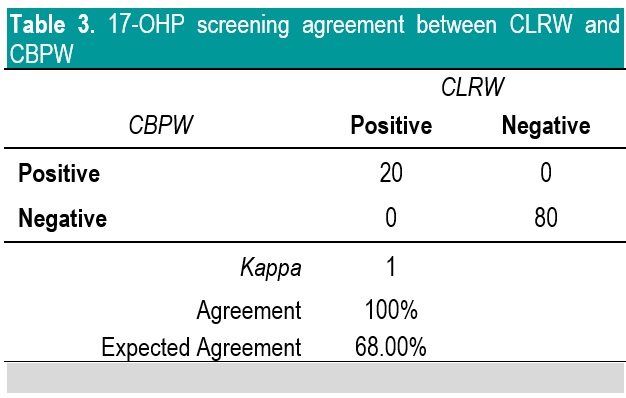
Table 3. 17-OHP screening agreement between CLRW and CBPW
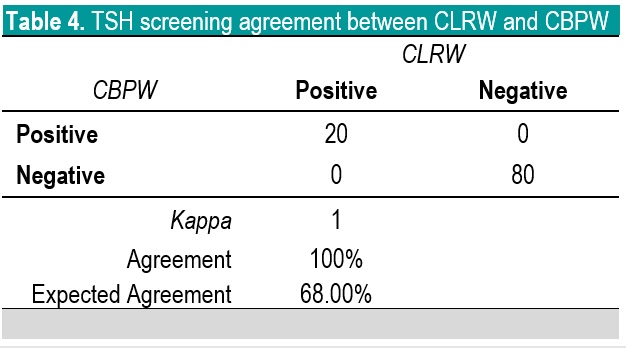
Table 4. TSH screening agreement between CLRW and CBPW
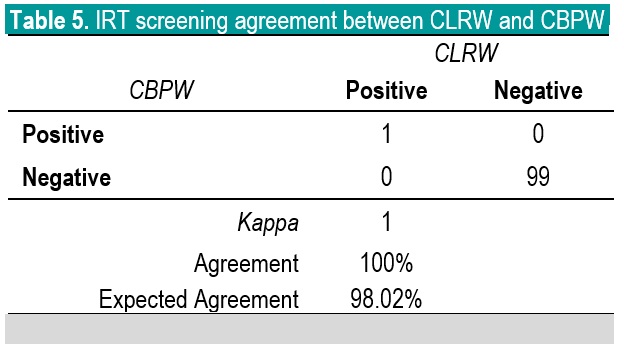
Table 5. IRT screening agreement between CLRW and CBPW
The parallel testing showed that overall, the performance of using CBPW in automated time-resolved fluorescent immunoassay for TSH, 17-OHP, and IRT is acceptable, compared with using CLRW as instrument feed water.
Time-resolved fluorescent immunoassay is widely used for measurement of various hormones in biological specimens.16 As a solid-phase method, a washing step is needed in order to remove unbound analytes, and unbound labeled indicator reagents that may create background noise to the signal detected by the instrument.17 CLRW are used as instrument feed water in automated instruments for this purpose.14
CBPW may have met the specifications for CLRW when it was bottled by the manufacturer, however, it is recommended that laboratories must validate that the bottled water is fit for its intended purpose in their setting.14
In this study, we have validated the use of CBPW, and have observed that it has no significant difference in the performance of automated time-resolved fluorescent immunoassay for TSH, 17-OHP, and IRT compared with using CLRW as instrument feed water.
Based on our findings, we conclude that CBPW can be used as substitute to CLRW as instrument feed water in automated time-resolved fluorescent immunoassay of TSH, 17-OHP, and IRT in NBS using AutoDELFIA (Perkin-Elmer).
All authors certified fulfillment of ICMJE authorship criteria.
The authors declared no conflict of interest.
None.
[1] Padilla CD. Newborn screening in the Philippines. Southeast Asian J Trop Med Public Health. 2003;34:87-8. PubMed
[2] Padilla CD, Therrell BL. Newborn screening in the Asia Pacific region. J Inherit Metab Dis. 2007;30(4):490-506. PubMed CrossRef
[3] Desai MP, Sharma R, Riaz I, Sudhanshu S, Parikh R, Bhatia V. Newborn Screening Guidelines for Congenital Hypothyroidism in India: Recommendations of the Indian Society for Pediatric and Adolescent Endocrinology (ISPAE) - Part I: Screening and Confirmation of Diagnosis. Indian J Pediatr. 2018;85(6):440-7. PubMed CrossRef
[4] NSRC. Fact Sheet: Information for DOCTORS about the disorders included in the Expanded Newborn Screening Panel. Manila: NSRC; 2016. >https://www.newbornscreening.ph/images/stories/ResourcesTechnicalDocuments/Expanded%20Screening%20Fact%20Sheets_Doctors_2015.pdf
[5] White PC. Update on diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Curr Opin Endocrinol Diabetes Obes. 2018;25:178-84. PubMed CrossRef
[6] Ross LF. Newborn screening for cystic fibrosis: a lesson in public health disparities. J Pediatr. 2008;153(3):308-13. PubMed NIHMSID: NIHMS67805 PubMed Central
[7] Wilcken B, Wiley V. Newborn screening. Pathology 2008;40(2):104-15. PubMed CrossRef
[8] Witchel SF. Newborn screening for congenital adrenal hyperplasia: beyond 17-hydroxyprogesterone concentrations. J Pediatr (Rio J) 2018;pii: S0021-7557(18)30712-5. PubMed CrossRef
[9] Kopacek C, Prado MJ, da Silva CMD, et al. Clinical and molecular profile of newborns with confirmed or suspicious congenital adrenal hyperplasia detected after a public screening program implementation. J Pediatr (Rio J) 2018;pii:S0021-7557(17)30413-8. PubMed CrossRef
[10] Slagle KM, Ghosn SJ. Immunoassays: tools for sensitive, specific, and accurate test results. Laboratory Medicine. 1996;27:177-83. CrossRef
[11] Self CH, Dessi JL, Winger LA. Ultra-specific immunoassays for small molecules: roles of wash steps and multiple binding formats. Clin Chem. 1996;42(9):1527-31. PubMed
[12] Kocova M, Anastasovska V, Sukarova-Angelovska E, Tanaskoska M, Taseva E. Clinical practice: experience with newborn screening for congenital hypothyroidism in the Republic of Macedonia - a multiethnic country. Eur J Pediatr. 2015;174(4):443-8. PubMed CrossRef
[13] al Saedi S, Dean H, Dent W, Stockl E, Cronin C. Screening for congenital adrenal hyperplasia: the Delfia Screening Test overestimates serum 17-hydroxyprogesterone in preterm infants. Pediatrics. 1996;97(1):100-2. PubMed
[14] CLSI. Preparation and Testing of Reagent Water in the Clinical Laboratory: Approved Guideline. CLSI document C3-A4. Fourth ed. Wayne, Pennsylvania: CLSI; 2006.
[15] DOH. Philippine National Standards for Drinking Water of 2017. AO 10 s 2017. https://www.doh.gov.ph/node/12121.
[16] Ito K, Goto T, Tsuji A, Maeda M. Time-resolved fluoroimmunoassay for pituitary adenylate cyclase activating polypeptide 27 (PACAP27) using europium (III) ion chelate labeled streptavidin-biotin complex. Journal of Pharmaceutical and Biomedical Analysis. 1997;15(9-10):1489-95. PubMed
[17] Cox KL, Devanarayan V, Kriauciunas A, Manetta J, Montrose C, Sittampalam S. Immunoassay methods. In: Sittampalam G, Coussens N, Brimacombe K, eds. Assay guidance manual. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2012.