The External Quality Assessment Scheme (EQAS) evaluates the performance of participating laboratories through an external agency by which known blinded samples are sent to participants for analysis, and their performance evaluated and monitored.
The Transfusion Transmissible Infections – National Reference Laboratory provides an external quality assessment scheme for transfusion transmissible infections to blood service facilities in the Philippines with the aim of raising the standards of quality testing in infectious diseases in blood units and as a mandatory requirement in the licensing of laboratories.
In the 2017 test event, 180 participants were given an EQAS panel composed of the HVHT4120 serology program and the MLRA415 malaria program. Results were submitted through an online informatics system managed by OneWorld Accuracy Canada using the ISO 13528:2008 Robust Statistics method (Huber’s Method). Results were analyzed and evaluated with the reference result of the NRL to which non-concordant results would be marked aberrant.
From the 14,392 generated results from the HVHT4120 program and 885 generated results from the MLRA415 program, 51 (0.35%) results and 86 (9.72%) results were reported as aberrant respectively. The aberrant results reported were either due to random or systematic errors.
Analyzed data from this test event are used for the continuous improvement of their competencies and the renewal of their license to operate as required by the Department of Health.
Key words: quality assurance, blood donor serology, transfusion transmissible infections, proficiency testing
The quality management system model developed by the Clinical and Laboratory Standards Institute (CLSI), lists assessment an important element of the 12 quality system essentials and defines it as a tool for examining laboratory performance and comparing it to standards, benchmarks or the performance of other laboratories.[1] An external quality assessment scheme (EQAS) is a method by which an independent external agency uses known samples with undisclosed results and is commonly used to establish inter-laboratory comparability.[2]
In the Philippines, participation in an external quality assessment scheme for transfusion transmissible infections is a mandatory requirement for the licensure of blood service facilities[3] and aims to raise the standards on the quality testing of blood units.
This activity evaluated the performance of the blood service facilities in the Philippines by analyzing the results of the external quality assessment scheme conducted by the Transfusion Transmissible Infections – National Reference Laboratory in 2017.
Panel Composition
The TTI EQAS 2017 test event consisted of two panels, the HVHT4120 for blood donor serology, and the MLRA415 for malaria slide microscopy. The HVHT4120 consisted of twenty (20) pooled plasma samples obtained from blood donors from different regions of the country. Each pooled sample was prepared by mixing similar volumes of at least two samples that had similar antibody and antigen profiles. All samples were subjected to filtration prior to aliquoting. The samples were aliquoted, and their homogeneity confirmed. The serology profile for HIV, HBV, HCV, Syphilis of each sample were identified using a chemiluminescence assay (ChLIA), enzyme immunoassay (EIA), Rapid Plasma Reagin (RPR), Particle Agglutination (PA) and a Differentiation/Supplemental Assay (SA).
Program code MLRA415 consists of five (5) blood smears. The samples were obtained from Malaria patients in Palawan and prepared by the NRL for Malaria and other Parasites of the Research Institute for Tropical Medicine
Participants
The multimarker blood serology EQAS panel ID HVHT4120 and malaria microscopy EQAS panel ID MLRA415 were distributed to 180 participants nationwide. These participants enrolled for the EQAS 2016 test event with a corresponding registration fee to cover expenses for the test event.
Majority of the participants were private institutions (44%) followed closely by government institutions (42%) and the remainder are from the different Philippine Red Cross chapters (14%). Figure 1 shows the distribution of participants by region.
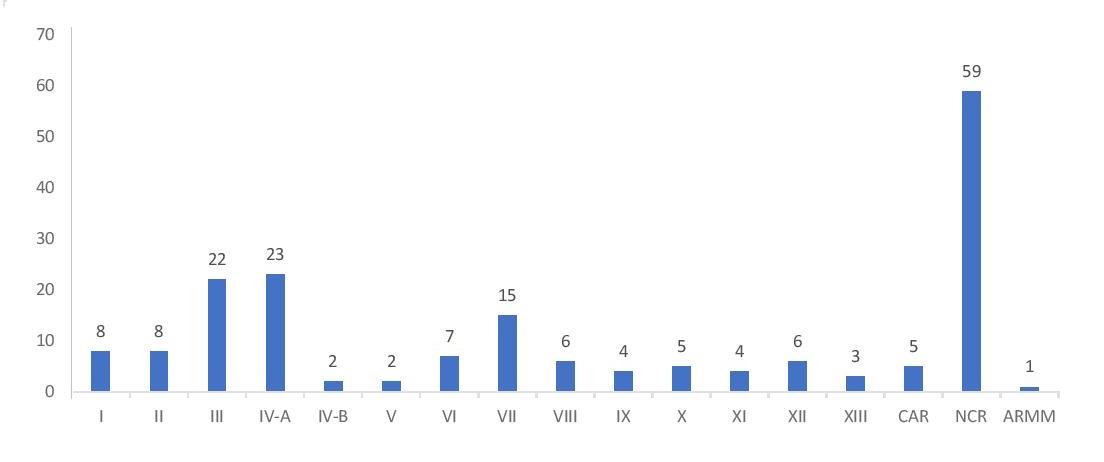
Figure 1. Regional distribution of participants.
Data Analysis
ISO 13528:2005 Robust Statistics method (Huber’s Method) was used to identify outlying results (numerical test results found to be statistically different from other test results reported by participants that tested the same sample in the same assay) for the created peer groups. A peer group is defined as a set of laboratories that utilize the same test format and assay test kit for screening TTI. The said method uses the mean as an estimator and outlying test results were removed from statistical calculation. Qualitative results of the BSF were compared with the qualitative reference results of the NRL Discrepancy between the two results would mark a result aberrant.
A total of 14,392 results were generated from 75 assays for the HVHT4120 panel and 885 results were generated from 1 assay for the MLRA415 panel.
Data entry errors: Two participants reported a “reactive” test result but submitted a “negative” assay interpretation.
False positive results: Nine participants reported false reactive results on known negative samples.
False negative results: Five participants reported false negative results on initial testing.
Educational sample (HIV and HCV): Two participants reported false negative results on the HIV and HCV sample with one of the participants having reported a “reactive” test result but submitted a “negative” assay interpretation. One participant had reported a reactive HBsAg result.
Educational sample (HIV p24 Antigen): Two participants reported a “reactive” result using a 3rd generation HIV assay. Eleven participants reported a “negative” result using a 4th generation HIV assay with one participant having reported a “reactive” test result but submitted a “negative” assay interpretation. Three participants reported an “inconclusive” test result using a 4th generation HIV assay. Three participants reported a reactive HBsAg result on the HIV p24 antigen sample.
Of the total number of results generated in the HVHT4120 panel, 51 results (0.35%) were reported as aberrant.
On rating the performance of the participants, the following criteria must be met to be classified as an unsatisfactory performer in the HVHT4120 initial panel:
(a) at least one false negative result;
(b) at least twenty percent (20%) false positive results.
In accordance with these criteria, corresponding participants were given an investigation checklist to assist them in identifying errors and make the necessary corrective actions and/or troubleshooting methods. A 2nd set of the HVHT4120 panel were given to participants for retesting if the identified unsatisfactory performance was due to a testing error. Participants with aberrant results due to transcription errors were only given an investigation/troubleshooting checklist and a written recommendation. Three (10) participants were given a second set of samples wherein one participant had reported a false negative result and one participant did not submit their results.
Of the total number of results generated in the MLRA415 panel, 86 results (9.72%) were reported as aberrant.
Figure 2 shows the distribution of grades of the participants. They have been evaluated and graded as follows:
- Excellent – 100% acceptable results on the initial panel (all final results were correctly identified in comparison with the reference results);
- Very Satisfactory – Less than 100% acceptable results on the initial panel without being given a second panel for retesting.
- Satisfactory – 100% acceptable results on retesting of the second panel; or had an aberrant result in the initial panel due to a clerical error, given that the participant was able to identify this error through the EQAS investigation checklist.
- Poor – Participant did not follow minimum requirements of testing as per DOH Circular No. 2013-0132 or less than 100% acceptable results on retesting of the second panel; or had an aberrant result in the initial panel due to a clerical error which the participant had failed to identify in the EQAS investigation checklist.
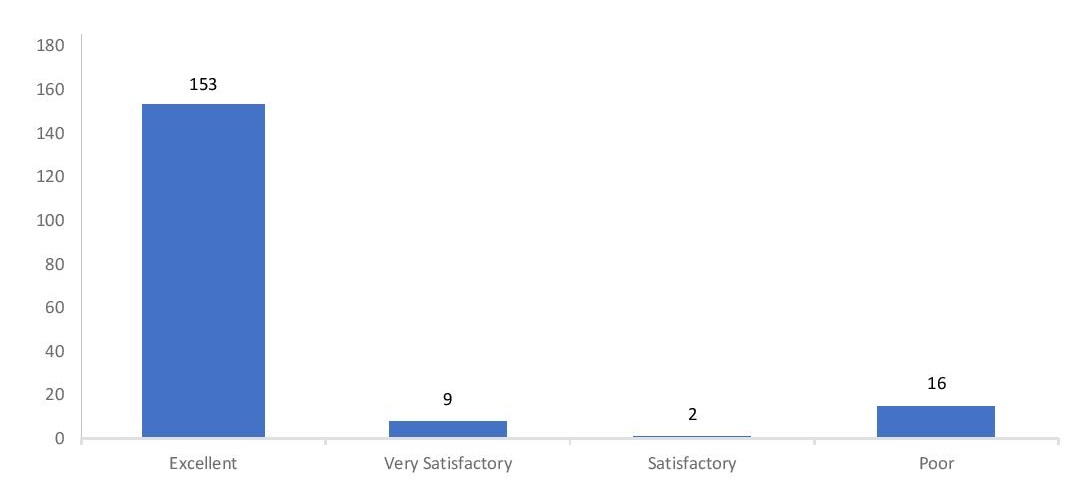
Figure 2. Distribution of grades for the EQAS 2017 test event.
EQAS is an essential element of the quality system and plays a vital role in facilitating optimal patient care.[4] The transfusion transmissible infections EQAS directed for blood service facilities was designed to assess the entire phase of testing and monitor the quality of laboratory results. This also enables the participants to compare their performance with other laboratories and this can aid them in detecting potential problems which present opportunities for improvement.
The participants should regularly review their results as part of quality improvement regardless of their rating. Participants should take responsibility in implementing the necessary corrective action as part of the quality assurance program in their laboratory.[5]
The authors thank the TTI-NRL staff, Dr. Catherine Masangkay and Dr. Socorro Lupisan of the Research Institute for Tropical Medicine (RITM), the Health Facility Development Bureau, the Health Facility Services and Regulatory Bureau, the Department of Health – National Voluntary Blood Services Program, the National Council for Blood Services – Technical Committee, the Department of Parasitology (RITM), Philippine Red Cross – National Blood Center (Port Area), Asian Hospital and Medical Center, OneWorld Accuracy – Canada and Joe Vincini from NRL – Australia.
We would also thank all participating Blood Service Facilities for their support.
All authors certified fulfillment of ICMJE authorship criteria.
The authors declared no conflict of interest.
None.
[1] World Health Organization. Laboratory Quality Management System. Handbook. Geneva, Switzerland: WHO Press, World Health Organization, 2011.
[2] World Health Organization. Quality assurance in bacteriology and immunology, 3rd edition. New Delhi, India: Publishing and Sales, World Health Organization, Regional Office for South-East Asia, 2012. Retrieved from http://apps.searo.who.int/PDS_DOCS/B4871.pdf?ua=1
[3] Department of Health. DOH Department Memorandum No. 2009-0086B: Amendment to Department Memorandum No. 2009-0086-A entitled, “Implementation of external quality assessment program as regulatory requirement for licensing of clinical laboratories.” 8 September 2014. Retrieved from http://ttinrl. com/wp-content/uploads/2014/11/DM-2009-0086-B.pdf
[4] Sciacovelli L, Secchiero S, Zardo L, Plebani M. External quality assessment schemes: need for recognised requirements. Clin Chim Acta. 2001;309(2):183-99. PubMed CrossRef
[5] Frean J, Perovic O, Fenshan V, et al. External quality assessment of national public health laboratories in Africa. 2002–2009. Bull World Health Organ. 2012;90:191-9A. CrossRef